7.1. Limiting specific nutrient
7.2. Effect of protein: Quantity and quality
7.3. Theoretical model for P:E ratio
Taken together, the evidence shows that the tissue gained during catch-up growth is of variable composition, with a tendency towards a limitation of lean tissue deposition. The basis for the limitation is not clear, and theoretically may be primarily of either a nutritional origin, or of a constitutional or regulatory nature. An increase in dietary protein of itself does not appear to improve the rate of protein deposition, and it may possibly be an effect that is secondary, a consequence of some other limiting nutrient.
It can be stated, therefore, that an adequate intake of energy is a necessary, but not sufficient requirement for adequate catch-up growth. There is a need to identify and define the relationship between energy and nutrient intake: is it fixed or is it variable?
There is evidence to suggest that under optimal conditions lean tissue is deposited in preference to adipose tissue (RUDMAN et al., 1975). This makes sense teleologically, providing the useful working hypothesis that the finding of an impairment of lean tissue repair implies a limited availability of one or more nutrients or metabolic substrates. GOLDEN and GOLDEN (1981) have found that when children who had recovered from severe malnutrition received oral supplements of zinc, there was an increased rate of weight gain, for the same dietary intake of energy and protein, with a decreased cost of growth. This shift towards lean tissue deposition with supplemental zinc would suggest that zinc may be one of the factors which limit lean tissue growth.
The study of nitrogen balance has been the standard approach to determining the protein requirements during catch-up growth. Nitrogen balance is essentially a measure of the relationship between the intake of protein and the urinary excretion of urea (other N components of urine being more or less unchanged). The assumption has been made that the urea excretion reasonably approximates urea production. If neither of these assumptions were justified, then we would need a major reappraisal of the approach. Studies in vitro and in vivo have shown that the rate at which the liver synthesises urea is determined by the rate at which the tissue is presented with a suitable nitrogenous substrate, and the relative competition for that substrate between the needs for tissue synthesis and urea synthesis. If the pattern of amino acids available is poorly suited to the pattern needed for the proteins being formed by the tissue, then those amino acids present in excess of needs are oxidised and the nitrogen goes to form urea (HARPER, BENEVENGA and WOHLHUETER, 1970). This is the basis of the principle of protein quality. Hence the rate of urea production is a direct indication of the quality of the protein. Therefore, by following the relation between the protein supplied and the urea produced, it is possible to deduce the potential for utilising the available protein for tissue synthesis. This is the basis for using nitrogen balance and weight gain as indicators of the rate of growth.
It is now generally accepted that in normal adults, on an adequate protein intake of 85 g/d, only 70 to 80% of the urea produced each day is excreted in the urine, with the remaining 20 to 30% being hydrolysed in the bowel with the nitrogen being made available for further metabolic interaction (WALSER and BODENLOS, 1959; JACKSON, PICOU and LANDMAN, 1984). On a low-protein diet of 35 g/d, there is an increase in the relative distribution of the urea produced, with 60 to 70% of production being retained (LANGRAN, MORAN and JACKSON, unpublished observations). In absolute terms, the potential saving of nitrogen on a low-protein diet represents about 50 g protein/d. The synthesis of each molecule of urea requires at least 4 ATP; the energy cost of reutilising the urea nitrogen is not known. Walser has characterised this process of urea hydrolysis and reutilisation as a futile cycle, and it certainly is energetically costly. As pointed out by WEBSTER (1988) the concept of a 'futile' cycle is not helpful if the cycle has some purposive or useful function. It is likely that the reutilisation of urea nitrogen is of substantial functional significance (JACKSON, 1989).
During catch-up growth, the ability to utilise dietary protein effectively for the deposition of lean tissue is the primary determinant of the rate at which recovery can proceed. If dietary protein is used effectively, then the rate of urea synthesis should be low (STEPHEN and WATERLOW, 1968). If the pattern of amino acids in the protein is of poor quality in relation to the proteins that need to be synthesised, then urea production should increase. If the overall demand for protein by the body is high relative to the dietary protein intake (c.f. a low-protein diet), then the rate at which urea nitrogen is salvaged and reutilised for urea synthesis should increase.
7.2.1. Constant energy intake and variable dietary protein
Two groups of children were studied during catch-up growth. They both received 711 kJ (170 kcal) metabolizable energy/kg per day, but the level of protein was varied between the two diets: 4.5 and 3.7 g protein/kg per day, resulting in P:E ratios of 10.6 and 8.8% (JACKSON et al., 1990). There was a significant difference in the rate of weight gain between the two diets, 18 g/kg per day versus 12 g/kg per day, respectively. Expressed as a cost of growth, this represented a significant difference in the proportion of lean tissue being deposited, 60% on the higher protein intake compared with 32% on the lower. It may be concluded that the rate of weight gain is determined by the level of protein in relation to energy in the diet.
Urea kinetics were measured in all the children. There was no significant difference between the diets in the rate of urea production, but on the low-protein diet only 38% of the urea produced was excreted, compared with 62% on the higher protein intake. The protein equivalence of recycled urea was 0.77 g protein/kg per day on the higher protein intake and 1.53 g protein/kg per day on the lower intake, 17 and 40% of the intake.
An alternative approach to the data might be to assume that the energy cost of maintenance is the same regardless of the diet, and also that for both diets there is a similar pattern of tissue deposition. If this were true, then there would be a theoretical wastage of energy on the low-protein diet of about 96 kJ (23 kcal)/kg per day which might be associated with the recycling of urea.
7.2.2. Variable energy intake and constant protein
A comparison can be made between the studies of JACKSON, PICOU and LANDMAN (1990) and PICOU and PHILLIPS (1972).
In both studies, children were given 3.7 g protein/kg per day during rapid catch-up growth. In the study of PICOU and PHILLIPS (1972), the energy intake was 669 kJ (160 kcal)/kg per day (a P:E ratio of 9.25%), whereas JACKSON, PICOU and LANDMAN (1990) gave 711 kJ (170 kcal)/kg per day (a P:E ratio of 8.8%).
PICOU and PHILLIPS (1972) found that the dietary protein was utilised relatively efficiently with urea production being 53% of the dietary nitrogen intake (P/l). An increase in the metabolizable energy by 42 kJ (10 kcal)/kg per day reduced the efficiency of utilisation of dietary protein with P/l increasing to 80%. Whereas on 669 kJ (160 kcal)/kg per day children excreted 60% of urea production in the urine, on 711 kJ (170 kcal)/kg per day excretion was reduced to only 36% of production. Therefore, the children receiving 711 kJ (170 kcal)/kg per day behaved as though they were receiving an inadequate supply of protein, having activated the urea salvage mechanisms, not seen on 669 kJ (160 kcal)/kg per day. Thus, a change in energy intake of 6% around a P:E ratio of about 9% was sufficient to induce a switch in the response, suggesting the operation of a mechanism that is extremely sensitive. We are unable as yet to define either the signal for the response, or the detailed mechanism through which the response is activated.
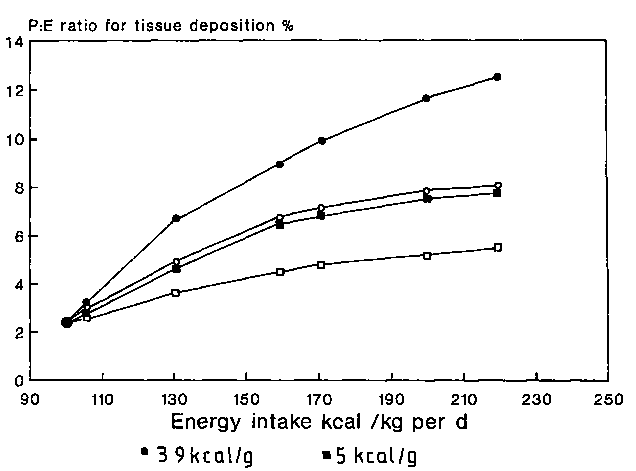
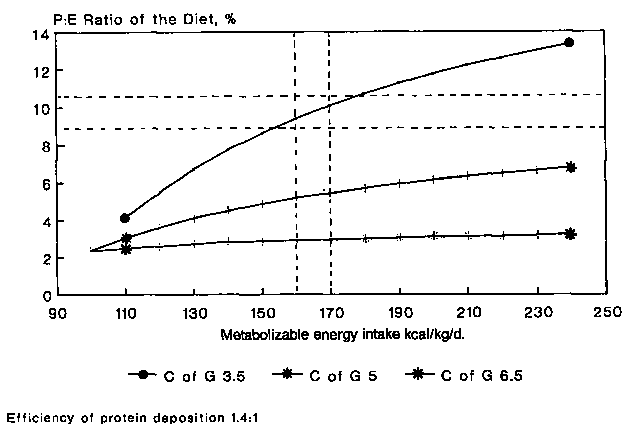
It is possible to model a theoretical relationship between the dietary intake of energy and protein in relation to the rate of weight gain and the type of tissue that forms that weight gain (see MILLER and PAYNE, 1963). It is reasonable to assume that during catch-up weight gain the maintenance requirement for dietary energy is 420 kJ (100 kcal)/kg per day (to cover the energy cost of basal metabolism, activity, thermogenesis and tissue deposition) (KERR et al., 1973). The maintenance requirement for protein is 0.6 g/kg per day (CHAN and WATERLOW, 1966). If the energy content of the weight gained is 21 kJ (5 kcal)/g, then each g of tissue would contain 0.1 g protein. Therefore on an energy intake of 439 kJ (105 kcal)/kg per day, 0.7 g protein/kg per day would be required to deposit 1 g/kg per day of new tissue. Hence, the P:E ratio of the dietary intake would be 2.7%. Table 3 shows how for an increasing energy intake, and hence an increased rate of weight gain, the P:E ratio would increase to allow for protein tissue to constitute the same proportion of the total weight gain. This relationship presumes that the protein synthesised would be deposited with an efficiency of 100%. Figure 3 shows the same relationship derived in Table 3 and also shows the relationship that would obtain if 1.6 g of protein had to be synthesised for every 1 g of protein deposited. Also shown in Figure 3 is the relationship that one would obtain if the protein content of the tissue deposited were increased to 60% of the total weight. It can be seen that there are at least five factors acting independently and together, which determine the relationship between the quality of the tissue deposited and the dietary energy and protein: (1) absolute energy intake, (2) relative energy intake, (3) absolute protein intake, (4) protein intake in relation to the energy intake, and (5) the efficiency with which protein that has been synthesised is deposited. Figure 4 shows three situations in which the tissue deposited contains 22, 44 and 66% of lean tissue to demonstrate the substantial influence that the P:E ratio of the diet has upon the ability to deposit lean tissue.
Table 3. During rapid catch-up weight gain the ability to deposit lean tissue is determined by the dietary availability of energy and protein. On the assumption that the maintenance requirement for energy is 420 kJ (100 kcal)/kg per day, and that for protein is 0.6 g/kg per day, the protein:energy ratio for maintenance would be 2.4%. If the energy content of the weight gained is 21 kJ (5 kcal)/g, then the protein content of the weight gained would be 0.1 g/g. Based upon these assumptions it is possible to calculate a theoretical P:E ratio for the diet to enable different rates of lean tissue gain to be sustained
Intake |
Weight gain |
P:E ratio |
|
Energy kcal/kg/d |
Protein g/kg/d |
g/kg/d |
% |
100 |
0.6 |
nil |
2.4 |
105 |
0.7 |
1 |
2.7 |
130 |
1.2 |
6 |
3.7 |
160 |
1.8 |
12 |
4.5 |
170 |
2.0 |
14 |
4.7 |
200 |
2.6 |
20 |
5.2 |
220 |
3.0 |
24 |
5.5 |
7.3.1. Constant energy and protein, and variable quality of energy
Three groups of children were given diets that provided 711 kJ (170 kcal)/kg per day, with 3.7 g protein/kg per day, to give a P:E ratio of 8.8%. The diets were based upon an infant milk formula, but the source of additional dietary energy varied between the three diets. In one group, oil was added, in the second oil, cornstarch and sucrose, and in the third oil and pectin. Added oil provided 40% of the total energy in the final formulation of the first diet; the cornstarch and sucrose provided 20% of the total energy in the second diet, and the pectin provided 3% of the total energy in the third diet.
The rates of weight gain on the first and second diets, 14 and 12 g/kg per day were not significantly different from each other. The addition of pectin reduced the rate of weight gain to 7 g/kg per day. If it is assumed that there is an equivalent energy cost for maintenance on each of the three diets, then the cost of weight gain would be 21, 24 and 42 kJ (5, 5.8 and 10 kcal)/g for diets 1, 2 and 3, respectively, i.e., 44, 32 and 0% of tissue deposited as lean. If, on the other hand, it is assumed that the tissue deposited in each case is balanced, and that the composition of this tissue is similar to that laid down when 711 kJ (170 kcal)/kg per day of a diet with a P:E ratio of 10.6% is given, then one has to be able to account for the disappearance of the excess energy not stored. The energy unaccounted for, expressed as a percentage of the energy available for growth (293 kJ (70 kcal)/kg per day) would be: for fat 22%; for CHO 23%; and for pectin 61%. Pectin is said to be extensively fermented in the large bowel and does not act as a faecal bulking agent. If the unaccounted for energy represented faecal loss as bacterial mass, then the mass of the stool would increase by the equivalent of 84 kJ (20 kcal)/kg per day. If the pectin is to be considered as available energy, the diet had a P:E ratio of 8.8%. If the energy in pectin is not available, then the ratio would be 9.1%.
The implication of these observations is that, during catch-up in weight, the form in which the energy is taken in the diet and the relationship between the absolute amount of energy to that of other nutrients makes a significant difference to the rate of weight gain and the pattern of tissue deposited during that weight gain. There are very few studies in which the level of dietary control has been sufficiently stringent to allow one to make definitive comments about the energy requirements for catch-up weight gain. The finding that the addition of pectin to the diet had such a marked effect upon the weight gain, either due to the deposition of excessive adipose tissue, or the wasting of energy, either through metabolic wasting or faecal losses, causes one to consider with care how one treats the dietary fibre in a consideration of energy intake. Once again the literature is far from clear in providing help in interpretation. There are reports which imply that the bulking effects of vegetable-based formulations, or the beneficial effects of the short chain fatty acids derived from fermentation may have negative or positive effects upon the quality and rate of weight gain. There is clearly a need for more precise information derived from studies in which the diet is controlled with care, and in which the outcome variables are identified specifically.
8.1. Colonic fermentation
8.2. Energy from SCFA
8.3. Factors influencing SCFA production
8.4. Gross versus metabolizable energy
8.5. Faecal energy and non-starch polysaccharide
8.6. Faecal energy in cystic fibrosis
During the past decade there has been an increased interest in the effects of dietary fibre on human metabolism and physiology. It is now widely appreciated that substantial fermentation of dietary carbohydrate takes place in the large intestine, but the contribution made by fermentation to the energy economy of the body is less clear. Fermentation is an important component of normal large-bowel activity (CUMMINGS and ENGLYST, 1987). It is the process whereby anaerobic bacteria (and yeasts) break down dietary and other substrates, principally carbohydrate, to obtain energy for growth and the maintenance of cellular function. A variety of potentially fermentable substrates may enter the large intestine. These include dietary fibre (non-starch polysaccharide, NSP), unabsorbed dietary residue (starches and sugars) and endogenous secretions and cellular debris (intestinal glycoproteins and mucopolysaccharides). The relative proportions of each of the substrates will vary with dietary intake, the extent of maldigestion or malabsorption and mucus production within the gut. The end-products of anaerobic carbohydrate breakdown are short-chain fatty acids (SCFA: acetic, proprionic and butyric acids), gases (hydrogen, carbon dioxide and methane) and energy. The energy is used by the intestinal microflora for growth, whereas most of the SCFA are absorbed through the colonic mucosa and may contribute to the host's energy supply. The output of SCFA in faeces is minimal (RUBENSTEIN, HOWARD and WRONG, 1969). The absorption of SCFA stimulates sodium and water absorption, thereby being an important contributor to salt and water homeostasis in the colon. The production of SCFA may be of importance in maintaining the health of the epithelium of the large bowel. The epithelial cells of the colon metabolize SCFA, especially butyrate, which is their preferred fuel (ROEDIGER, 1980). Following absorption, SCFA pass into the portal vein and thence to the liver where proprionate and some acetate are taken up. The remaining acetate may pass on to peripheral tissues, being metabolized by muscle.
The total production of SCFA in the human intestine is unknown. The factors which influence both production and absorption have yet to be established. Attempts have been made to estimate SCFA production from a stoichiometric appraisal based on the formula for fermentation derived by MILLER and WOLIN (1979):
34.4 C6H12O6 (r) 64 SCFA + 34.23 CO2 + 10.5 H2O
It can be calculated, using molar ratios, that approximately 10 mmol of SCFA will be produced for each gram of carbohydrates broken down in the human colon, yielding approximately 12 kJ (3 kcal) as available energy. Consequently, even modest amounts of fermentation from dietary fibre alone would contribute to metabolizable energy intake (e.g., 10 g/d carbohydrate fermentation would yield 120 kJ (30 kcal)/d in the form of SCFA).
McNEIL (1984) has calculated the amount of carbohydrate fermented and SCFA produced on the basis of the growth requirements of intestinal bacteria (approximately 0.1 mol ATP is needed to generate 1 g dry weight of bacteria). As faeces from adults on a British diet contain an average of 15 to 20 g bacteria (dry) (STEPHEN and CUMMINGS, 1980), approximately 1.5 to 2.0 mol ATP would be needed to replace the daily faecal output of bacteria. As 1 mol hexose yields 5 mol ATP when metabolized anaerobically, 50 to 65 g of hexose are required to produce 1.5 to 2.0 mol ATP. Of this, 10 to 15 g may be derived from dietary NSP, the remaining 35 to 50 g must be derived from unabsorbed starches and sugars or endogenous secretions. The yield would be approximately 500 to 600 mmol of SCFA, with a total energy value of 600 to 750 kJ (144 to 180 kcal)/d. This represents approximately 75% of the original energy content of the carbohydrate; the remaining 25% may be used by the colonic microflora for growth. This kind of calculation has led to the view that between 6 to 10% of daily energy needs could be met by fermentation of the fibre typically consumed in the Western World.
The extent of the production of SCFA and the contribution to metabolizable energy intake appears to depend on several factors.
Firstly, the amount of dietary fibre and the degree to which it is digested. There has been considerable interest in the digestibility of different forms of dietary fibre within the human large intestine (CUMMINGS, 1984). However, the extent to which microbial fermentation takes place and the extent to which the end-products of fibre fermentation are absorbed and contribute to metabolizable energy intake remain an area of controversy. Increases in microbial cell excretion were observed in association with an increased intake of vegetable fibre from cabbage whereas the increased consumption of the much less digestible wheat fibre resulted in only a small change in faecal microbial excretion (STEPHEN and CUMMINGS, 1980). Thus, the higher the fibre intake, particularly that derived from beans, vegetables and fruits, the greater the potential contribution from colonic fermentation.
Secondly, the amount of unabsorbed carbohydrate delivered to the large intestine. Clearly, this may be increased as a result of maldigestion and malabsorption associated with disease (e.g., cystic fibrosis or lactose intolerance). However, it is now clear that some foodstuffs contain relatively large amounts of starch in a form that is resistant to digestion - both in vitro and by human digestive enzymes. A number of studies have demonstrated that not only does the resistant starch largely escape digestion in the human small intestine, but that other types of starch may also pass into the large intestine to undergo fermentation (CUMMINGS and ENGLYST, 1987).
Thirdly, the quantity and nature of endogenous material delivered to the large intestine is not clear. Mucus degradation by colonic microflora has been well documented, and the presence of bacterial sub-populations that produce extracellular glycosidases with the specific role of degrading complex oligosaccharides of mucin in the gut lumen have been identified (HOSKINS and BOULDING, 1981). It has not been possible to directly quantify mucus production and epithelial cell losses, hence the extent of the contribution made by fermentation of mucopolysaccharides and glycoproteins to metabolizable energy remains unknown. However, in circumstances where mucus production is substantially elevated (e.g., in cystic fibrosis), the potential capacity for energy to be salvaged might be substantial (see later).
Finally, what is the overall magnitude of the gross energy intake and to what extent can the intake satisfy the energy needs of the individual? The relative contribution made by colonic fermentation may be relatively small when the gross energy intake is high. In contrast, for individuals on a marginal intake of energy, or in whom requirements are elevated, the relative contribution will be greater and may be critical.
Further support for the role that colonic fermentation may play in meeting the energy needs of the individual comes from balance studies in which the true metabolizable energy intake (gross intake minus faecal and urinary losses) has been compared with the metabolizable energy intake estimated from food composition tables. There are several approaches in use for calculating the metabolizable energy content of mixed diets from the foods and the nutrients they contain. In 1899, ATWATER and BRYANT published factors that could be applied to the protein (4 kcal/g), fat (9 kcal/g) and carbohydrate (4 kcal/g) content of different foods based upon balance experiments in human subjects. The factors were subsequently modified by MERRILL and WATT (1973) who stated that if the revised factors were used, the deviation between the true and calculated metabolizable energy intake would not exceed 5% of the true value for most diets. In the United Kingdom, a slightly different approach has been used (PAUL and SOUTHGATE, 1978). The methods differ in the approach adopted for the calculation of metabolizable energy from carbohydrate. Paul and Southgate gave the metabolizable energy as 'available carbohydrate' (effectively excluding NSP) x 3.75 kcal/g. In contrast, MERRILL and WATT (1973) used a range of values, 3.87 to 4.12 kcal/g for carbohydrate. Using the first approach, SOUTHGATE and DURNIN (1970) found that the contribution of dietary NSP to metabolizable energy could be disregarded on diets containing low levels of NSP, up to 32 g/d.
GÖRANZON and FORSUM (1986) showed that when the potential contribution made by dietary fibre (NSP) was excluded there was a consistent underestimation of the metabolizable energy for diets high in dietary fibre. They calculated that dietary fibre, derived mainly from cereals, would contribute 10 kJ (2.5 kcal)/g to metabolizable energy. The dietary fibre from beans, vegetables and fruits would provide 13 kJ (3.1 kcal)/g. These values are in general agreement with the findings from studies in ruminants where approximately 70-75% of the heat of combustion of NSP may be available for metabolism (WHISKER, MALTZ and FELDHEIM, 1988). If these figures are applicable in humans, the energy available from dietary fibre would be a maximum of 13 kJ (3.1 kcal)/g (CUMMINGS, 1981).
Whilst there is evidence that NSP may contribute to the metabolizable energy of an individual, there is also evidence that increasing the amount of NSP in the diet may also lead to an increased excretion of fat, nitrogen and energy; the result being a decrease in the apparent digestibility of fat and protein and a reduction in available energy (SOUTHGATE and DURNIN, 1970; WISKER, MALTZ and FELDHEIM, 1988). In these studies, the total increase in energy losses associated with the increased intake of NSP exceeded the gross energy contained in the NSP itself. On the basis of these observations, FAO/WHO/UNU (1985) have proposed that no extra correction needs to be made to the metabolizable energy derived using Atwater factors, when the diet contains small amounts of dietary fibre. With increasing amounts of dietary fibre the calculated metabolizable energy should be reduced by about 5%. It is proposed that metabolizable energy may need to be further reduced when the consumption of dietary fibre is high: of the order likely to be ingested in many developing countries. This recommendation should not be applied uncritically, without an improved understanding of the potential contribution made by colonic fermentation to metabolizable energy and the nature and origin of the energy within the stool.