App 1.1: Single pool systems
App 1.2: Systems with two or more pools
Contributor: Andy Coward
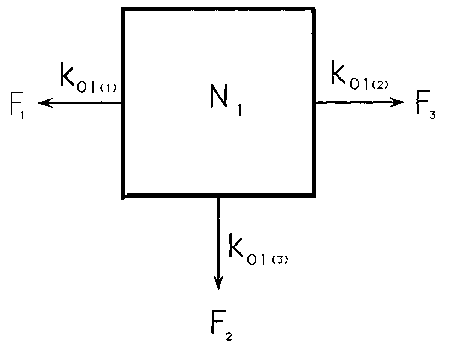
Figure App 1.1 illustrates a single pool of water
with volume N and input and output rates equal at a value F per unit time. The fraction of
the pool being replaced per unit time is thus F/N and we call this value the rate constant
for the system (k).
If a quantity (Qo) of a tracer is added to this at time zero, calculus shows that:
Qt = Qoe-kt
If N remains constant we can convert this equation for quantity of tracer into one for tracer concentration (C):
![]()
Ct =Coe-kt

N = pool space
F = flow
k = rate constant where subscript 01 indicates to
the outside (O) from compartment 1.
Symbols are as in Figure App 1.1.
Symbols are as in Figure App 1.1.
Thus, the slope of a plot of log Ct against time has the value k and the time zero intercept is Co. The quantity of tracer added to the system Qo is the dose given (A) in the context we are discussing here so we can calculate N as A/Co.
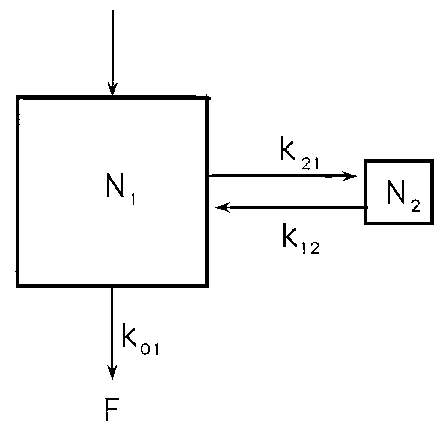
The single pool system we have
described has only one exit to the outside. If we consider other exits, as illustrated in
Figure App 1.2, it is important to realise that the value k represents the sum of all the
fractional losses (k = k1 + k2 + k3) and cannot be used
to obtain values for individual exits. These would need to be directly investigated. This
leads us to an important concept in analysis of this type, and that is, that the precise
nature of the system cannot be deduced unambiguously from tracer data and only if a model
is correct will values calculated from tracer data be correct.
Figure App 1.3 illustrates a simple two compartment system. Let us suppose that N1 is body-water and N2 is some other pool with which the 2H or 18O in water exchanges. In this case if enough observations were made on the isotopic concentration in body-water it would be seen that the disappearance curve consists of two exponential components that add together to produce the observed curve. However, the slopes of these exponentials are not directly equivalent to k01 or any of the other rate constants in the model system. Similarly, the intercepts of the individual exponentials do not directly give the values of N1, N2 or (N1 + N2) x N1 is in fact given by the sums of the intercepts.
As far as the present application of the tracer methodology is concerned we have therefore to consider what is likely to happen if a single-compartment solution is applied to a system that is in reality a two-compartment one. For example, in the system in Figure App 1.3 we can assign values of 0.1 and 0.005/day for k01 and k21 respectively and vary k12 from 5 to 0.00005, calculate the characteristics of the curve and then fit a single compartment model to it using early concentration values from Days 0 to 14. With this sampling regime there would be no convincing evidence that a two compartment system existed. Table App 1.1 shows the results. For rapid recycling of isotope predicted volume (N1) and rate constant (k01) are close to true values and inversely related so that little error is incurred in the measurement of flow (F) as the product (k01)(N1). However, as the degree of recycling becomes smaller errors in k01 increase because we reach the stage when the estimate of N1 is correct (recycling is so slow that N1 cannot see N2), but k01 is overestimated by 5% as a consequence of the virtually unrecycled losses as k21. The latter case is what is likely to happen to some extent when 2H is sequestered as fat (see Chapter 7). Elsewhere (Chapter 4) it is suggested that rapid recycling of 2H explains the differences between volumes obtained with 18O and 2H.
Table App 1.1. Effect of different degrees of isotope recycling in a two-compartment system when a one-compartment solution is used
k12 |
N1 |
k01 |
F |
5 |
1.0011 |
.09986 |
.09997 |
0.5 |
1.0066 |
.09960 |
.10026 |
0.05 |
1.0051 |
.10284 |
.10336 |
0.005 |
1.0007 |
.10472 |
.10479 |
0.0005 |
1.0000 |
.10499 |
.10499 |
0.00005 |
1.0000 |
.10499 |
.10499 |
Assumptions used:
k01 = 0.1
k21 = 0.005
k12 = variable
N1 = 1
FTrue = (k01) (N1) = 0.1