Introduction
Biomedical mechanisms
Hormonal regulation of fetal growth
Nutrition and placental functions
Maternal environment
Maternal disorders
Maternal nutrition and iugr
Interaction factors
References
Discussion
Reference
JA Prada and RC Tsang
Correspondence: Dr JA Prada
Children's Hospital Medical Center, P.O. Box 670541, Cincinnati, Ohio 45267-0541, USA
The causes of intrauterine growth retardation (IUGR) are multiple, involving many different factors. Studies in humans and animals have shown that the maternal environment is the most important determinant of newborn weight, accounting for more similarity in birth weights of siblings than does genetic affinity. In addition to a direct relationship with the degree of maternal plasma volume expansion, many clinical factors are associated with IUGR. These factors include multiple gestation; fetal, genetic, and chromosomal anomalies (Down's syndrome and Turner's syndrome); infections such as TORCH syndrome (acronym for toxoplasmosis, rubella, cytomegalic disease, and herpes); and various maternal disorders including anemia, severe chronic asthma, chronic renal disease, heart disease and hypertension. Maternal stress factors, including narcotic addiction, cigarette smoking and chronic alcoholism, are associated with IUGR. Placental anomalies including hemangiomas, placental infarcts, single umbilical artery, and small placental size are also associated with intrauterine growth retardation. Poor nutritional status of the mother at conception and inadequate energy and protein intakes during pregnancy can also result in IUGR. Because IUGR children are not a homogeneous group, they have a broad spectrum of growth, health, and developmental outcomes. In general they have higher rates of subnormal growth, morbidity, and neurodevelopmental problems. The biomedical mechanisms reflected in nutritional, infection-related, hormonal, and metabolic parameters are not likely to be independent causative factors of IUGR, but important mediating factors of a pathologic process set in motion by other agents and insults. This paper focuses mainly on the possible negative effects that a deficient maternal diet might have on fetal development and growth.
Antenatal diagnosis of intrauterine
growth retardation (IUGR) is based on our limited understanding of the stages of fetal
growth. In the first and second trimester, placental growth is primarily due to
hyperplasia, while hypertrophy predominates in the late third trimester (Winick, 1971).
Fetal growth, in contrast, is solely due to hyperplasia (Low and Galbraith, 1974). Fetal
length increases most rapidly towards the end of the second trimester, while weight gain
is primarily a phenomenon of the third trimester. More specifically, body length increases
most rapidly around 20 weeks of gestation (Streeter, 1920). Fat makes up only about 1% of
total body weight at 26 weeks of gestation, but 12% at 38 weeks (Widdowson, 1968). Any
type of deprivation or insult occurring early in gestation could therefore affect both
length and weight by interference with early hyperplasia in all body organs. A similar
insult occurring late in the second trimester would exert its maximum effect on body
length. In the third trimester body weight would primarily be affected (Villar and
Belizan, 1982).
Biologically, maternal weight and nutritional status are determinants of the environment in which conception is to occur, a certain amount of body fat being required for normal reproductive function. Epidemiological data show associations between early menarche, low pre-pregnancy weight, low pre-pregnancy height, and short inter-pregnancy interval on the one hand, and downward shifts in growth curves or increased risk for growth retardation in subsequent pregnancies on the other (Schell and Hodges, 1985; Cnattingius S et al, 1984; Miller and Merritt, 1979; Tanner and Thomson, 1970; Ferraz et al, 1988; Miller, 1989; Yang et al, 1989). Mothers born with low birth weight are more likely to produce low birth weight infants (Klebanoff et al, 1989).
The role of genetic determinants
Maternal genetic variations are likely to affect fetal growth. There are differences in growth curves in different geographic regions and among different racial groups in the United States. Kessel et al (1988) and Myers and Ferguson (1989) reported that, after correction for social and demographic factors, blacks still have a higher rate of fetal death and are at increased risk for delivering premature and SGA infants. However, it is extremely difficult to separate environmental factors from true genetic differences, and these data should be interpreted cautiously.
Growth in utero is the result
of cell multiplication and tissue organization; the effects of genetic endowment on size
at birth are small. This is demonstrated by the calculated correlation coefficient between
length at birth and adult stature, which is 0.3. In the two-year-old the correlation
coefficient between height and adult stature is quite high, 0.8 (Battaglia and Meschia,
1986). It then declines again in the early teenage years with the pubertal growth spurt.
Continued decline of percentiles after the age of two years, even in apparently healthy
children, should be cause for further investigation.
The hormonal regulation of fetal growth is complex. During pregnancy, hormones appear to be important mediators of substrate availability to the fetus. Growth depends on the stage of gestation, and nutrient availability. A normal balance of the functional unit, consisting of uterus, placenta and fetus, is of great importance for fetal growth. Late in gestation some maternal physical factors might constrain fetal growth, as demonstrated by embryo transplants and crossbreeding experiments (Gluckman and Liggins, 1984; Snow, 1989). A common illustration is the reduced mean birth weight in multiple pregnancies.
Fetal growth might be controlled at the level of individual cells and organs by nutrient supplies and/or by locally active factors. For example, IGFs have been described as acting on cell growth via autocrine or paracrine mechanisms (Adamson, 1993; D'Ercole, 1991). Stimulation of mitosis appears to be a major function of IGF-2; its absence results in IUGR mice (DeChiara et al, 1990). IGF-1 is also capable of inducing cell differentiation (Ohlsson et al, 1993). Pituitary growth hormone (GH) is found in the fetal circulation by 12 weeks of gestation (Cornblath et al, 1965). Despite its abundance early in the second trimester, the role of GH in intrauterine growth is not clear. Pituitary aplasia and congenital hypopituitarism do not cause severe IUGR (Lovinger et al, 1975; Goodman HG et al, 1968). Children with abnormal GH receptors, however, are usually short at birth (Laron et al, 1972). GH seems to be involved in the initiation of synthesis of IGF-binding protein, which plays a major role in fetal growth. Pancreatic agenesis is associated with severe growth retardation (Lemons et al, 1979), and fetal hyperinsulinemia leads to fetal mass overgrowth.
IGF-1
Circulating levels of IGF-1 in fetal, and cord blood correlate with fetal size (Lassare et al, 1991). Reduced plasma concentration of IGF-1 has been reported in IUGR (Ashton et al, 1985). When mouse embryos with high plasma IGF-1 concentration are transplanted into unselected maternal recipients, intrauterine growth is larger than that of transplanted embryos with low concentration of IGF-1 (Gluckman et al, 1992). Maternal starvation leads to a rapid decrease in fetal IGF-1 concentration, which is generally associated with the cessation of intrauterine growth (Basset et al, 1990). Glucose is the major regulator of fetal IGF-1 secretion (Oliver et al, 1993).
The above fetal determinants might act independently or in conjunction with maternal determinants. During pregnancy, the maternal hormonal profile might be modified by placental hormones such as human chorionic somatomammotropin. Maternal IGF-1, IGF-2, and insulin do not cross the placenta, and do not have a direct effect on fetal growth, but may have an effect on placental function, thus altering the nutrient exchange between the placenta and the fetus. For example, administration of IGF-1 to pregnant rodents eliminates the maternal physical constraint on fetal growth and alters the relationship between fetal size and placental size (Hall K. et al, 1984). Maternal plasma IGF-1 concentration correlates with fetal growth (Mirlesse et al, 1993; Smith et al, 1992).
The placenta is also an active endocrine organ, secreting steroids and polypeptide hormones. The placenta synthesizes estrogen, and progesterone (Simpson and MacDonald, 1981) and a number of other growth factors involved in autocrine and paracrine mechanisms of fetal development. Recently, the placenta has been shown to express the GH-V gene specifically leading to the production of a placental growth hormone (placental GH) (Chen et al, 1989). Early in pregnancy (15-20 weeks of gestation) pituitary GH is present in the maternal circulation. Later in pregnancy (20 weeks to term) increased placental GH replaces pituitary GH (Frankenne F et al, 1990). Placental GH declines rapidly with the onset of labor and after delivery. Plasma samples of mothers of IUGR babies contain significantly lower concentrations of placental GH (Mirlesse et al, 1993); plasma levels of IGF-1 are also reported to be low, suggesting a relationship between placental GH and the development of the feto-placental unit. However, since placental GH is not detected in the fetal circulation, it does not appear to have a direct effect on fetal growth.
Human placental lactogen (hPL) is detected by six weeks of gestation and its concentration increases progressively throughout gestation (Handwerger S, 1991). hPL stimulates insulin secretion, and causes nitrogen retention during pregnancy (McGarry and Beck, 1972). hPL is also lipolytic, and thus, might help to maintain glucose availability to the fetus during maternal starvation (Walker et al, 1991). However, there is no direct evidence of a role of hPL in fetal growth.
The role of infection
Acute infections may affect the fetus temporarily because of maternal pyrexia Chronic infections may act on the fetus by crossing the placenta, and directly altering fetal cell growth. A few infecting agents may interfere with the utero-placental transfer mechanism and reduce the supply of nutrients. Where malaria is endemic, it is one of the most common causes of IUGR. McGregor et al reported a 20% infiltration of the placenta with extensive villus damage, and reduced birthweight (McGregor et al, 1983).
Cytomegalovirus infection is
commonly associated with congenital abnormalities of the fetus, and 40% of the infants
born with this condition have IUGR (Stagno et al, 1983). Rubella might limit cell
multiplication in the fetus, and damage the vascular endothelium of the villus
capillaries, thus impeding normal circulation. Cooper et al found that 60% of
infants with congenital rubella had birth weights below the tenth centile. Other virus
infections, such as herpes and hepatitis, have occasionally been reported to be associated
with IUGR (Waterson, 1979).
The placenta has multiple functions that are very important for normal fetal growth. For example, the placenta consumes up to 50% of oxygen and glucose extracted from the uterine circulation. When substrate availability is reduced, the placenta reduces the consumption of oxygen and glucose and increases the consumption of amino acids (Owens et al, 1989), establishing a complex balance of nutrient utilization between itself and the fetus. This is achieved by the fetus becoming catabolic and providing the substrate needed for a placental oxidative metabolism.
Placental transport of nutrients to the fetus
All of the nutrients used by the fetus for energy production and growth are transported by the placenta (Battaglia and Meschia, 1986). Some (e.g. glucose and fatty acids) are transported by facilitative transport proteins according to maternal-to-fetal concentration gradient kinetics. Others, like amino acids, are transported by active energy-dependent transporter proteins. Gases are transported by passive diffusion. Many other nutrients (e.g. lactate and ammonia) are products of placental metabolism.
Glucose is the most important carbohydrate transported to the fetus by the placenta. Smith et al (1992) reported that this transport is accomplished by the GLUT 1, a facilitative transporter protein that shows specificity for glucose among hexoses. Simmons et al (1979) showed that the arterial plasma glucose concentration gradient from the mother to the fetus is the physiological driving force that determines placental glucose uptake and transfer to the fetus. The capacity for placental transfer increases with gestational age (Molina et al, 1991, Figure 1), as does the placental concentration of GLUT 1 transporter (Morris et al, 1985). If hypoxic stress (hypertension) or persistent placental hypoglycemia (maternal starvation) is present, fetal catecholamine secretion may promote glucogenolysis and decrease fetal insulin concentration as well as glucose utilization. In prolonged hypoglycemia even fetal glucogenolysis has been reported (Narkewicz et al, 1993). Hypoxic or hypoglycemic states could result in IUGR.
Nitrogen is supplied to the fetus by placental transport of amino acids. Amino acid transport occurs by energy-dependent processes via amino acid transport proteins (Yudilevich and Sweiry, 1985). The placenta does not just function as an amino acid pump; rather, it selectively takes up, metabolizes and transports each amino acid individually (Carter et al, 1991). This is one of the most important advances in our understanding of placental amino acid transport, providing evidence of the fundamental role of the placenta as a metabolic regulator of fetal nutrient supply. Such a regulatory role probably has important implications for growth and protection of the fetus during critical developmental periods. For most of the amino acids, concentrations in fetal plasma exceed those in maternal plasma. Based on this energy-dependent condition, it is not surprising that experimental maternal hypoxemia in animal models results in decreased transport of some amino acids to the fetus (Milley, 1988). Studies in pregnant sheep also indicate that amino acid transport to the fetus may be limited when uterine blood flow (UBF) is reduced chronically, as may be the case during hypertension that occurs during pregnancy (Lang et al, 1994), or when the mother is made chronically hypoglycemic (Carver et al, 1993).
The human placenta also appears to have a great capacity for lipid transfer, using transporters for specific fatty acids and more complex pathways. These pathways include lipoprotein dissociation with placental lipoprotein, lipase and triglyceride uptake and metabolism (Coleman, 1986). Lipids are released into the fetal plasma as free fatty acids, or lipoproteins. In humans, fetal patterns of essential fatty acids and structural lipids correlate directly with the fatty acid-lipid composition of the maternal plasma, and thus, with the maternal diet (Davis, 1923). However, the role of placental lipid metabolisms in fetal growth is unknown.
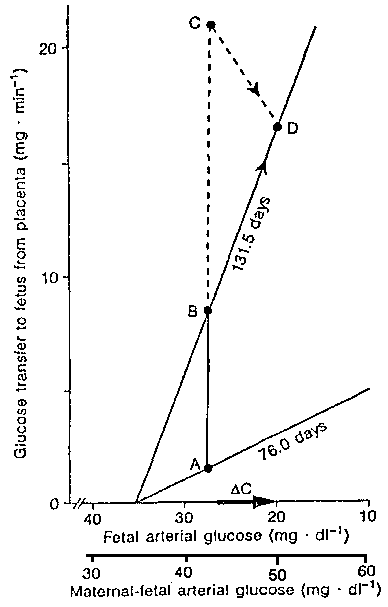
The glucose transfer capacity
increases with gestation (slope at 76.5 days, compared with slope at 131.5 days).
Placental glucose transfer increases with gestation (points A and B). As fetal growth
continues there are additional demands for glucose (point C). The actual glucose supply to
the fetus is illustrated at point D. (From: Molina RA et al, Am J Physiol
261:R697-R704, 1991).
There is an association between parity and birth weight. Primigravidae are more likely to give birth to small for gestational age babies than multiparous women. In the British Births Survey of 1970 (Chamberlain et al, 1975) firstborn babies were lighter than babies born from multiparous mothers, especially babies born after less than 37 weeks of gestation. Moreover, Billewics and Thomson have shown an increase in birth weight over the woman's whole reproductive life (Billewicz and Thomson, 1973). Carr-Hill and Prithchard (1985) however, reached the conclusion that increases in birth weight from one child to the next was more closely related to maternal weight before successive pregnancies than to parity.
In a pregnancy with a normally grown fetus the maternal plasma volume increases by about 50% with possibly an additional 5% in multigravidae. This increase and the birth weight are directly related (Hytten and Paintin, 1963). In fetal growth retardation the increase in plasma volume may be only half of that in a normal pregnancy. The increase in plasma volume is least in those who have a history of recurrent abortion, or IUGR. If plasma volume expansion is less than normal but the red cell volume increases normally, then the plasma viscosity rises. This increase of plasma viscosity is not linear but exponential at high haematocrits. The consequence of this is a reduced capillary flow and increased tendency to thrombosis. A maternal hemoglobin concentration over 12g % can result in placental infarcts and IUGR (Naeye, 1977).
About a third of multiple pregnancies finish preterm. In addition these babies also show IUGR compared with singleton babies. Monozygotic twins tend to be more growth retarded than dizygotic twins (Bulmer, 1970). A similar association has been reported in mono-amniotic twins (Wharton et al, 1968).
With increasing height above sea level, the atmospheric pressure is reduced and therefore the partial pressure of oxygen decreases. Sabrevilla et al (1968) showed a marked reduction in birth weight in babies born at extremely high altitudes. McCullough et al (1977) found a greater proportion of babies with IUGR among those born at a high altitude in the Rocky Mountains than among babies born in Denver, and Gibson et al (1973), showed an inverse relationship between mean birth weight and hemoglobin levels in pregnancy. High hemoglobin levels were associated with low increases in plasma volume, and this may be the real reason for the negative association between altitude and fetal growth.
Finally, one of the strongest
indicators of a multiparous woman's performance is her previous obstetrical performance.
It is conceivable that the increased risk of producing recurrent IUGR exists because of
characteristics of the mother rather than an assigned medical reason.
Hypertension
Probably the best identified cause of IUGR in the Western World is hypertensive disease. Some workers estimate that hypertensive conditions are responsible for up to a third of all fetal growth retardation. Pregnancy-induced hypertension (PIH), particularly if associated with proteinuria and/or pre-eclampsia, entails a greater risk of IUGR, and a longer duration of hypertension results in a higher degree of IUGR.
Numerous explanations have been proposed to account for the development of PIH, including incompatibilities between maternal and fetal blood types, high salt intake, calcium deficiency, poor general nutrition, changes in the renin-angiotensin system and predisposing genetic makeup. Several recent studies have linked high blood pressure and pre-eclampsia to hypocalcemia, but the specific role of calcium itself in the development of hypertension during pregnancy is not clear. Dietary intake of calcium appears to be an important variable in determining the incidence of hypertension during pregnancy. For example, an increased incidence of high blood pressure during pregnancy has been reported in parts of the world where dietary intake of calcium is low (Belizan and Villar, 1980), and conversely, in areas with high dietary calcium intakes the incidence of hypertension is low (Belizan et al, 1988).
Our studies (Prada et al, 1992,1994) of dietary calcium intakes during pregnancy illustrate the close relationship between diet, blood pressure, cardiac output, and uterine blood flow (Figures 2 and 3). These studies have found that maternal hypocalcemia is directly correlated with increased blood pressure, reduced cardiac output, and uterine blood flow in pregnant ewes. The fetuses of hypocalcemic and hypertensive mothers show reductions in blood ionized calcium concentration, PaO2, pH, and O2 content. Chronic reductions in uterine blood flow have been reported to affect fetal growth. A recent US multicenter clinical calcium trial during pregnancy, however, has shown no effect of calcium supplementation in preventing preeclampsia in healthy nulliparous women (Levine et al, 1997).

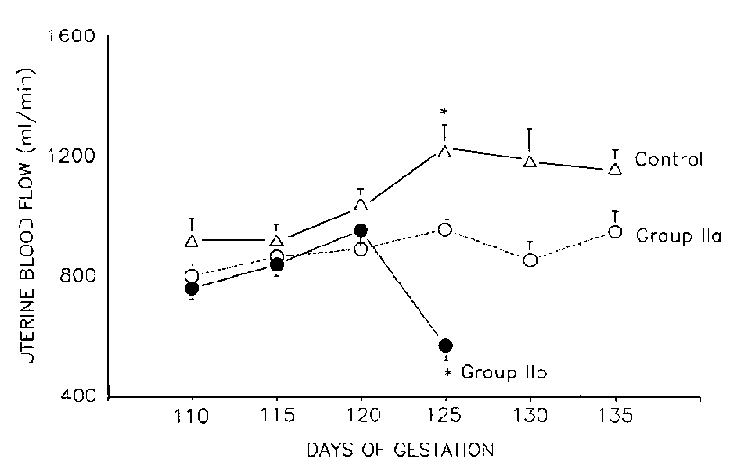
Experimental animals were placed on a calcium deficient diet from 90 ± 2 days of gestation to teen. Values are mean ± SEM. * significant difference from control (p=.01, Newman-Keuls test).
The chronic maternal diseases most likely to reduce fetal growth are renal disorders. Renal disease is associated with IUGR and the risk is increased if associated with hypertension. With nephrosclerosis about 9% of the fetuses are growth retarded. With a combination of glomerulonephritis and hypertension (pre-eclampsia) the proportion rises to 46% (Lin et al, 1982).
Cigarette smoking
The maternal behavior which most affects fetal growth is smoking, including passive smoking (Yerushalmy, 1971; Rubin et al, 1986). In 1958, Butler and Bonham (1963) showed an association between reduced birth weight and cigarette smoking. This association is dose-related, i.e. a function of the number of cigarettes smoked by the mother. Women who stop smoking before the 16th week of gestation have babies with similar birth weight patterns as nonsmokers. Multi-variant analyzes have shown smoking to be independently related to the incidence of IUGR (Sexton, 1986).
Since passive smoking reduces birth weight, and since smoking mothers frequently are married to smoking husbands, this is a truly familial risk factor, even when the mother stops smoking. Some of the mechanisms through which cigarette smoking may affect birth weight are: reduced expansion of plasma volume, increased maternal plasma carbon monoxide, and as a consequence of it, increased fetal blood carbon monoxide, increased maternal blood viscosity, and as a consequence, increased fetal blood viscosity. Smokers also tend to drink more alcohol than non-smokers (Yerushalmy, 1971).
Alcohol
Prospective studies have shown a
dose-related increase in the number of IUGR babies with alcohol consumption. Hanson et
al (1978) examined data from pregnant women in 1970. A growth-retarding effect on the
fetus was usually found at a much lower level of alcohol consumption than that required to
produce fetal alcohol syndrome. Even moderate alcohol consumption during pregnancy clearly
has a negative effect on fetal birth weight. Additional relative risks of IUGR have also
been reported in opiate addicts (Ostrea and Chaver, 1979).
There is now retrospective and prospective evidence that poor maternal nutritional status at conception and inadequate maternal nutrition during pregnancy can result in IUGR. Efforts to improve maternal and fetal nutrition during pregnancy have focused on achieving appropriate energy intakes and ensuring that the intakes of specific nutrients are adequate to meet maternal and fetal needs. In considering the relationship between gestational weight gain and pregnancy outcomes, attention has centered on birth weight. One reason for this is that birth weight is the pregnancy outcome most frequently examined in epidemiological studies. But a more fundamental justification for the emphasis on birth weight is its widely recognized association with infant mortality and morbidity. In 1923 Davis reported that maternal weight gain during pregnancy could be used as an indicator of maternal nutritional status and that, in turn, maternal nutritional status influenced fetal growth. Mean birth weight increased with gestational weight gains, from approximately 3,100 g with a 7-kg gain, to about 3,600 g with a 13.6 kg gain. Several methodologic issues should be kept in mind before considering the relationship between weight gain and fetal growth. For instance, studies often differ in assessment of gestational age and in the definition of IUGR. A number of chromosomal and other congenital anomalies associated with growth retardation may lead to prognoses much worse than those for infants without those anomalies. Major congenital anomalies affect only a small percentage of IUGR infants but account for a disproportionate number of deaths. Ounsted et al (1981) reported that 6.9% of the IUGR infants in their study had anomalies, but that they accounted for 62% of all deaths.
In recent studies, IUGR infants have often been subdivided according to their body proportions, as defined by Rohrer's ponderal index (birth weight divided by the length cubed). Those with low ponderal indices are classified as disproportional (also referred to as asymmetric). Many investigators have reported higher neonatal mortality rates among disproportional IUGR infants (Guaschino et al, 1986; Hass et al, 1987; Hoffman and Bakketeig, 1984), but better early catch-up growth and better prognoses for long-term growth and development than for proportional IUGR infants (Fancourt et al, 1976; Harvey et al, 1982; Hill et al, 1984; Villar et al, 1984). Unfortunately, most studies in this area have not controlled for the severity of IUGR. Based on a meta-analysis (Kramer, 1987) of 61 English and French language studies published between 1970 and 1984, the average magnitude of the effect on mean birth weight in women with adequate weight-for-height is approximately 20 g per kg of gestational weight gain. The relative risk for IUGR in women with low (< 7 kg) total gestational weight gain is approximately 2.0. Low maternal weight gain can account for about one in seven cases of IUGR.
The type of IUGR most frequently found in developing countries is closely related to conditions of poverty, and chronic malnutrition of economically disadvantaged mothers, yet the nutrient intake of pregnant women has been assessed only in relatively few studies during the last decade. Reported mean daily energy intakes varied markedly, ranging from 1500 to 2800 kcal (Institute of Medicine, 1990). Much of this may just be a reflection of differences in methodology, maternal body size and activity level. On average in the USA, intakes of protein, riboflavin, vitamin B12, and niacin exceed the Recommended Dietary Allowance (RDA). However, some nutrients are consumed consistently in amounts substantially lower than the RDA (Rush et al, 1988). These include vitamin B1, B6, D, E, and folacin; iron, zinc, calcium and magnesium (Brennan et al, 1983; Butte et al, 1981; Krane, 1970; Baker et al, 1975).
Studies of vitamin profiles in 174 mothers and their newborns in the USA, revealed thiamine deficiency in a significant number of cases (Butterworth, 1990). Causes of thiamine deficiency in pregnancy include the following:
a) inadequate dietary intake,
b) increased dietary requirements,
c) hyperemesis gravidarum,
d) malabsorption,
e) alcohol abuse,
f) infections including HIV,
g) effects of drugs,
h) genetic factors (Butherworth et al, 1991; Roecklin et al, 1990).
In the 1960s, a high incidence of
IUGR was described in children born to alcoholic mothers. Thiamine deficiency in pregnant
rats results in severe IUGR (Fournier and Butterworth, 1990; Heinze and Weber, 1990). A
recent report in humans demonstrated low blood cell thiamine concentration in mothers with
fetuses with severe IUGR (Borle, 1981). The author speculated that thiamine-dependent
enzymes are important for the maintenance of cellular energy metabolism, for lipid
synthesis, and for nucleotide synthesis in the developing brain.
Fetal growth retardation is the consequence of complex pathology. The physiopathology of fetal growth retardation involves the fetus, the placenta, the mother, and a combination of the three. The complexity involves different reasons for the disturbed growth, acting independently or in combination. Interactions among maternal nutrition, placental dysfunction and hormonal regulation can be recognized. Some fetuses may grow abnormally because their cells are deprived of nutrients, some because they have a reduced number of cells, and others may grow abnormally because of a combination of the two. Some viral infections may affect cell replication, and chromosome breakage may occur in severe malnutrition (Naeye and Blanc, 1965; Betancourt et al, 1974).
Insulin is essential for normal
growth (Cheek and Hill, 1975). It facilitates transfer of amino acids into cells, and thus
protein production, and increases Krebs activity which induces fat deposition. In growth
retardation the islets of Langerhans are reduced in size, fetal insulin levels are
lowered, and fetal growth is further reduced (De Prins and Van Assche, 1982; Sherwood et
al, 1974). Mother and fetus meet in the intervillous space. The maternal blood is in
direct contact with the placenta. The effect of this blood supply on fetal nutrition
depends on feto-placental factors, the volume of maternal blood perfusing the villi, and
also on the content of that blood. These factors are related to how well the mother adapts
to the pregnancy and to her environment. Illness, malnutrition, and smoking may modify
adaptation. However, immunological interactions between the mother and the fetus may also
modify adaptation. Multigravid women tend to adapt to pregnancy better than those in the
first pregnancy. Since retarded fetal growth is a description rather than a diagnosis, a
single pathology is unlikely.