N. S. SCRIMSHAW (Chairman), B. R. BISTRIAN, O. BRUNSER, M. ELIA, A.A. JACKSON, Z.-M. JIANG, J.M. KINNEY, I.H. ROSENBERG and R.R. WOLFE
1. Effects of infections on nutritional status
2. Environmental ('tropical') enteritis
3. Other chronic infections
4. Energy vs protein requirements
5. Possible role of specific amino acids
6. Summary
7. Recommendations
References
1.1. Anorexia
1.2. Cultural and therapeutic practices
1.3. Malabsorption
1.4. Catabolic losses
1.5. Anabolic losses
1.6. Fever
1.7. Additional intestinal losses
It is well established that infections worsen nutritional status and that energy balance is decreased, although to a lesser degree than protein balance. However, the extent to which the protein/energy ratio required during treatment and recovery is affected by various diseases is more difficult to specify. This is because multiple mechanisms are involved, some of which affect protein and energy needs differently, and also because there are variations in the impact of various infections depending on their etiology, severity and duration.
By
analyzing the ways in which infections worsen nutritional status,
their differential effects on protein and energy metabolism can
be better understood. These include:
Experience with nitrogen
balance studies that have been disrupted by intercurrent
infections reveals a consistent decrease in food intake even when
efforts are made to maintain it. This is observed even with
asymptomatic infections including live virus immunizations. Not
only are protein and energy intakes affected but also those of
other nutrients.
In the case of young
children with diarrhea or febrile infection there is a strong
tendency of the mother to withdraw solid food and substitute thin
starchy or sugary gruels that are low in protein and caloric
density or to offer herbal decoctions of various sorts. In
Guatemala, for example, children with measles are frequently
given agua de tisana made from a local wild plant.
In field studies it is not possible to separate the effects of anorexia from those of deliberate withdrawal of food for cultural reasons. While consumption of food will be reduced by anorexia, purposeful withholding of food may have much greater impact. The average reduction associated with the presence of specific symptoms of infection in Guatemalan children was approximately 20% at all periods from 15 to 60 weeks of age (MATA et al., 1977; MARTORELL and YARBROUGH, 1983; MARTORELL, RIVERA and LUTTER, 1990).
In Matlab,
Bangladesh (HOYLE et al., 1980), caloric intakes in
children under five years of age were more than 40% lower during
the acute stage of diarrhea than after recovery. The differences
were greater with diarrhea caused by rotavirus and E.
coli than with cholera and Shigella. In Peru, caloric
intake decreased from 10 to 86% among breast-fed children with
diarrhea (BENTLEY et al., 1991).
The significant decrease in
absorption of nutrients with diarrhea is referred to above. In
the metabolic studies of INCAP protein absorption was generally
reduced 10 to 30% and rarely as much as 40%. However, the
frequency of diarrhea! disease in young children makes this a
significant contribution to the frequent periods of decreased
energy intake in young children due to infections. POWANDA (1977)
has summarized additional literature and attempted to quantitate
the range of malabsorption of protein, fat and carbohydrate with
diarrhea.
Despite the reduced absorption it is important to maintain food intake to the extent possible during bouts of diarrhea. CHUNG and VISCOROVA (1948) reported that the absorption of nitrogen in four children with diarrhea varied from 40 to 74% and that of fat from 39 to 67%. For two similar cases in which food was withheld, absorption percentages were mildly depressed for both nitrogen and fat. Data from ICDDRB for diarrhea due to rotavirus average 43% for nitrogen, 42% for fat, 74% for carbohydrate and 55% for total calories. Corresponding figures for diarrhea due to enteropathogenic E. coli and Shigella were slightly higher (MOLLA et al., 1982).
The range
of infections that are associated with malabsorption is wide and
most are common in developing countries. They include the
bacterial, viral and protozoan enteritides, intestinal parasites
such as hookworm, fish tapeworm, ascaris, strongyloides; and
systemic disorders such as measles, tuberculosis and
streptococcal infections. These infections act by shortening
intestinal transit time or by physical blocking of mucosal
surfaces.
A catabolic response occurs
with all infections even when they are subclinical and not
accompanied by infection (BEISEL et al., 1967; BEISEL,
1972; 1975; BEISEL and WANNEMACHER, 1980; KEUSCH and FARTHING,
1986). Under the stimulus of the release of interleukin-1 and
tumor necrosis factor by phagocytic cells, endocrine changes are
initiated that lead to the mobilization of amino acids from the
periphery, primarily from skeletal muscle. Amino acids, such as
those of the branched-chain group (BCAAs), are utilized as energy
sources leading to the synthesis of alanine or glutamine. BCAAs
are rapidly taken up by the liver and utilized for
gluconeogenesis, while alanine and glutamine serve as intestinal
fuels, for regulation of acid-base and for gluconeogenesis by the
kidney.
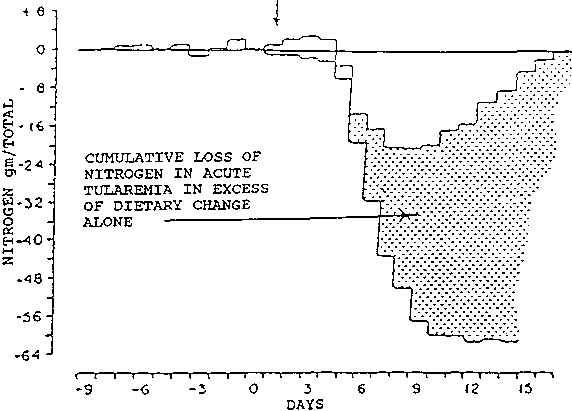
Amino acids, such as phenylalanine and tryptophan, which cannot be metabolized in skeletal muscle, are released in elevated amounts (WANNEMACHER, 1977). Figure 1 shows that in a young man with tularemia more than two thirds of the negative nitrogen balance was due to this metabolic response and the remainder to a spontaneous decrease in food intake (BEISEL et al., 1967). Figure 2 shows that even an individual with completely asymptomatic Q-fever can be in cumulatively increasing negative nitrogen balance for as long as 21 days (BEISEL et al., 1967).
In INCAP experience with metabolic studies in children, infections are always associated with a period of negative nitrogen balance, even in the case of immunization with yellow fever vaccine which provokes no symptoms (GANDRA and SCRIMSHAW, 1961). The response to these asymptomatic infections is qualitatively similar to those with the typical clinical disease.
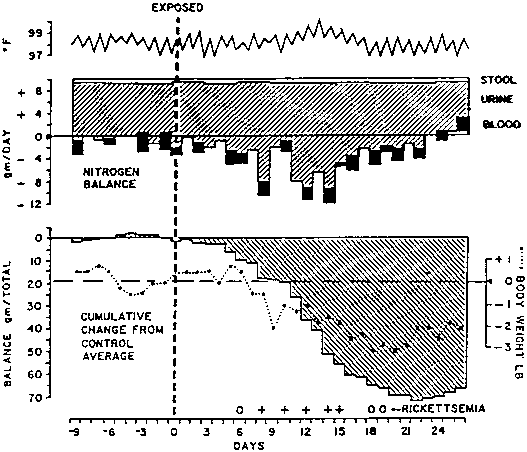
This individual showed neither an elevation of rectal temperature above 100° F nor diminution of dietary intake despite the presence of Coxiella burnetii in the blood over an 8-day period. (From BEISEL, 1977)
During an infection, amino
acids are diverted from normal pathways for the synthesis of
immunoglobulins, inflammatory cells, acute phase proteins and a
variety of other proteins including key liver enzymes
(WANNEMACHER, 1977). This would explain the common finding that
the extra nitrogen retained during the recovery period in
metabolic balance studies substantially exceeds that accounted
for by the magnitude of the negative balance during the acute
phase of an infection.
Whatever its benefits in
resisting infection, fever has a metabolic cost. The regulation
of normal body temperature within a narrow range is a complex
phenomenon that is modified by the endogenous pyrogenic activity
of interleukin-1, as well as other cytokines (tumor necrosis
factor, interleukin-6, interferon) released by mononuclear
leukocytes in response to infection and acting on the
hypothalamus. The resulting fever increases BMR by 13% for
each 1 °C (DuBOIS, 1937).
In some extended infections such as pneumonia and typhoid fever, the elevated temperature is maintained at a higher level with daily variations similar to those in the normal range. Conversely, in malaria there are sudden rises and falls in temperature. During the period of maximum fever, metabolism may increase by nearly one-third. Van't Hoff's law specifies that the coefficients for the increase in the velocity of various chemical reactions with temperature lies between 2 and 3. This means an increase in basal metabolic rate of 30 to 60% for a 3-degree rise from 37 to 40 (DuBOIS, 1936).
Since
carbohydrate stores are inadequate to meet the increased energy
requirement resulting from fever and the metabolic response to
infection (CAHILL, 1970), and since lipid stores are less
effectively used in the infected patient (BEISEL and WANNEMACHER,
1980), another source of energy is required. This is mainly
through gluconeogenesis from protein. Interleukin-1 releases both
insulin and glucagon from the endocrine pancreas and,
synergistically with tumor necrosis factor, stimulates muscle
breakdown (FLORES et al., 1989), thereby releasing
gluconeogenic precursor amino acids into the blood stream. As
already noted, the additional depletion of protein must be made
up during recovery. It should be emphasized that this process is
not dependent on fever, but the energy deficit is increased in
proportion to the degree and duration of the fever.
Difficult to measure
separately from malabsorption, but a significant additional cause
of further malnutrition, is the direct loss of nutrients into the
gut. Protein-losing enteropathy has been described for measles
(AXTON, 1975; DOSSETER and WHITTLE, 1975; SARKER et al., 1986),
diarrhea (WALDMAN, 1970; WHO, 1980; RAHMAN and WAHED, 1983) and
especially shigellosis (RAHMAN, ALAM and ISLAM, 1974). Alpha
1-antitrypsin is a simple and useful quantitative marker for
estimating the loss of protein into the gut in diarrhea! disease
(RAHAMAN and WAHED, 1983). It should be used for this purpose
with other infections with the potential to cause such losses.
In ICDDRB studies, nearly two-thirds of patients with enterotoxigenic E. coli and 40% of those with rotavirus diarrhea were also associated with excessive loss of protein in the feces. Between 100 and 500 mL of serum were lost with feces each day in patients with shigellosis due to protein-losing enteropathy.
Bleeding
into the intestine, from Schistosoma mansoni or hookworm,
also represents a loss of nutrients. BRISCO (1979) found that
each adult hookworm causes the loss of about 1 kcal/d. For an
estimated average hookworm load of 100 worms in ICDDRB studies
this would amount to an energy loss equivalent of 5 % of
adult caloric in takes. For mild-to-moderate infections, most of
the protein and about half of the iron loss due to bleeding into
the intestinal tract is reabsorbed. However, protein absorption
is significantly reduced with severe infections (DRAKE, 1959).
Some individuals may have as many as 500 worms.
The prevalence of
environmental enteropathy varies with the access of infectious
agents to the gastrointestinal tract. Between 30 to nearly 100%
of individuals living in an unsanitary environment experience
chronic changes in the intestinal epithelium that includes
blunting of the villi and increased numbers of lymphocytes in the
lamina propria progressing to a flat mucosa that resembles
celiac disease (BAYLESS, SWANSON and WHEBY, 1971; LINDENBAUM,
HARMON and GERSON, 1972). It has been reported for local
populations in Haiti (KLIPSTEIN et al., 1968), India
(BAKER, 1972; GORBACH et al., 1970), Thailand (SPRINZ
et al., 1962; KEUSCH, 1972), Bangladesh (LINDENBAUM, GERSON
and KENT, 1971), Colombia (MAYORAL et al., 1967; GHITIS,
TRIPATHY and MAYORAL, 1967), Puerto Rico (LUGO-DE-RIVERA,
RODRIGUES and TORRES-PENEDO, 1972) and undoubtedly occurs in most
developing countries. ROSENBERG, SOLOMONS and SCHNEIDER (1977)
showed that most rural Guatemalans had reduced absorption of both
protein and carbohydrate.
The acquired nature of the condition attracted considerable attention when it was identified in returning US Peace Corps volunteers, missionaries, and other expatriates who had lived in unsanitary environments (KLIPSTEIN and FALAIYE, 1969). Some individuals required up to a year for full recovery. Xylose and lactose malabsorption were later found in American military and Peace Corps personnel in Thailand (KEUSCH, PLAUT and TRONCALE, 1970).
In Chile,
BRUNSER et al. (1970) reported a fecal nitrogen loss in
young adults with mild environmental enteropathy of 16 mg/kg per
day when subjects were receiving an egg diet providing 0.8 g of
protein/kg, and the loss increased to 20 mg/kg when the diet
provided 1 g of protein/kg per day. Both diets provided 20 g of
insoluble fiber. A period of 3 months in a metabolic unit with
strict sanitation restored normal mucosa morphology.
Most acute infections are
self-limiting, and specific antimicrobial therapy now exists for
most infectious illnesses. For some diseases, however, clinical
recovery may be delayed and nutritional support becomes a
significant consideration. Persistant diarrhea, i.e., episodes
lasting longer than 15 days, follows from 10 to 20% of
acute episodes depending on the location (WHO, 1980).
The majority of these episodes do not have a dramatic symptomatology but are nevertheless associated with severe weight loss and malnutrition. Such individuals have considerable limitation in their capacity to digest and absorb nutrients, and some may require parenteral nutrition to stabilize and improve their nutritional status. However, it is important to feed them as much as possible by mouth, if necessary using predigested formulas or continuous tube feeding. The aim is to provide adequate nourishment to facilitate prompt recovery of the intestinal mucosa.
A certain proportion of children with acute diarrhea! disease of nonspecific cause have persistent carbohydrate intolerance and develop a much more severe and prolonged nutritional deficit (ROSENBERG and SCRIMSHAW, 1972).
There are some infections for which specific therapy is not effective and which have long-term and sometimes severe consequences for nutritional status. Most prominent of these is the current pandemic of Acquired Immunodeficiency Syndrome (AIDS). The malnutrition of AIDS has multiple etiologies. AIDS is a viral infection producing a systemic response that includes anorexia, fever and weight loss that initially responds to specific retroviral therapy with azothymidine (AZT) or dideoxyinosine (DDI). In addition to the adverse impact of the systemic response on nutritional status, involvement of the gastrointestinal mucosa with the virus may contribute further to the malnutrition (HUANG et al., 1988).
Patients with AIDS develop superimposed infections that do not normally produce symptoms in immunocompetent hosts. Gastrointestinal infections such as with Isospora Microsporidia, Mycobacterium avium intracellulare or cryptosporidia produce severe diarrhea and malnutrition in AIDS for which antimicrobial therapy is of limited effectiveness. A limited number of such patients (usually less than 10%) may benefit from home enteral or parenteral feeding (KOTLER et al., 1989; 1991).
There are a number of illnesses commonly seen in AIDS patients for which chemotherapy is more effective such as Pneumocystis carini, toxoplasmosis, cytomegalovirus, herpes simplex and tuberculosis. In these diseases the treatment in the hospital may be sufficiently long and difficult that enteral or parenteral alimentation may become necessary. This is especially likely in the patient who has an altered state of consciousness or requires mechanical ventilation.
A second
common clinical syndrome is that of nosocomial pneumonias or
intra-abdominal infections that develop in critically ill
patients following surgery or other critical conditions such as
pneumonia, major gastrointestinal bleeding, trauma, acute
inflammatory disease of the intestine and pancreas or chronic
organ insufficiency (liver, heart, lung, kidney). The organisms
often found in these situations are or become resistant to
multiple antibiotics. In such patients the clinical course may be
quite prolonged and the septic syndrome can be profoundly
catabolic. This general category of patient is the most common
indicator for parenteral and enteral nutrition in the hospital
setting.
In a previous IDECG
monograph, SRIMSHAW (1990) reviewed the available information on
the impact of infection on energy requirements. The protein
losses of 0.6 to 1.2 g of protein per kg of body weight described
by POWANDA (1977) would account for a concurrent energy loss of 7
kcal to nearly 30 kcal per kg of body weight. Reduced food intake
would further deplete an individual with acute infection, and the
consequences might be greater for protein than for total dietary
energy, because the diet is so often altered to one of lower
protein value during an infection.
The
difficulty with such calculations is that they do not include the
energy and protein expended for the multiple anabolic processes
associated with an infection. Balance studies suggest that these
must be substantial, but there are no quantitative estimates. The
best guide may be the relative amounts of protein and energy
required for catch-up growth following severe depletion. For a 7
kilo child (UNU, 1979) this ranges from 5.6% of protein calories
for a 10 g weight gain per day to 9.l% for a 90 g gain. For a
similar child with both infection and severe depletion,
the P/E ratios were calculated in the UNU report to be from 9 to
13% higher than for nutritional depletion alone, depending on the
assumed rate of repletion.
Studies suggesting that
branched-amino acids (BCAAs) may promote tissue anabolism have
led to considerable interest in the provision of BCAAs in total
parenteral nutrition (TNP). Their metabolic role as precursors
for the synthesis of muscle glutamine has also stimulated
interest in their use. Interactions between BCAAs and aromatic
amino acids in their transport into the brain, and the indication
that they may decrease brain tryptophan uptake and serotonin
production, has led to the suggestion that they may be of
therapeutic value. For example, they may be able to stimulate the
respiratory center and aid patients with sleep apnea.
Specific trials of TPN solutions enriched with BCAAs have shown the following relationships. In patients with hepatic-failure-induced encephalopathy, improvement of mental function is observed, but benefits over administration of similarly low amounts of regular amino acid mixtures are uncertain (NAYLOR et al., 1989). Trials of their influence on nitrogen balance in patients fed varying intakes of protein have shown:
(a) limited effects with intakes greater than 70 g,
(b) improvement at intakes between 40 and 70 g,
(c) no effect of intakes at less than 40 g, where other amino acids may be limiting.
In renal
failure, where lowering blood urea levels is desirable, enriched
mixtures containing 40 to 70 g total amino acids have been useful
in treatment.
Interest in glutamine has
developed over the last few years subsequent to developments in
two areas:
(a) the understanding of the metabolic importance of glutamine as a fuel for lymphocytes, macrophages and other rapidly dividing cells,
(b) understanding of the importance and regulation of the skeletal muscle glutamine pool.
Thus, as a potential fuel for the immune system, concern has been expressed that inadequate provision of glutamine from skeletal muscle as a result of stress from infections and burns may be deleterious (FURST, ALBERS and STEHLE, 1987; JEPSON et al., 1988; NEWSHOLME et al., 1988). Concern has also been expressed that the ability of the gut mucosa to act as a barrier to pathogens in sepsis or trauma may be limited by inadequate mucosal synthesis subsequent to reduced availability of glutamine (WILMORE et al., 1988; LACEY and WILMORE, 1990). Finally, the suggestion that glutamine in skeletal muscle may act to regulate protein balance in this tissue has also led to attempts to limit the negative nitrogen balance in trauma by the provision of glutamine (ABUMRAD et al., 1989; HAMMARQVIST et al., 1989).
In
parenteral nutrition solutions, the instability of glutamine
during autoclaving has resulted in its omission from such
solutions. Several authors have suggested the inclusion of
glutamine containing dipeptides in TPN solutions. The suggestion
has been made that enteral provision of glutamine could also be
advantageous. To date there is no quantitative information about
the dose-response of either immune function or gut-mucosal
function to provide a basis for recommendations.
Malnutrition occurs
commonly as a consequence of cancer and represents an important
prognostic sign. Response to treatment and survival are inversely
correlated to the degree of weight loss, although intestinal
obstruction may contribute to weight loss in some cancers. In
most instances, the development of malnutrition in cancer is a
systemic response to the tumor and is principally due to anorexia
and reduced intake rather than through changes in energy
expenditure. Therefore, protein and energy are more equally
depleted than with infection. However, similar to infection
(STREAT et al., 1987), nutritional repletion is
ineffective in cancer cachexia that develops as a systemic
reponse (NIXON et al., 1981).
The usual chemotherapy regimen for most tumors is of short duration, and although anorexia and weight loss commonly occur, chemotherapy does not elicit a severe catabolic response. Thus, it is not surprising that data from a number of randomized clinical trials investigating the role of invasive nutritional support during chemotherapy did not identify a net benefit (KLEIN et al., 1986).
In bone marrow transplantation, however, the chemotherapy regimens are much more aggressive and prolonged, and malnutrition resulting from these regimens can be clinically important. Nutritional support under these circumstances has been shown to benefit morbidity, mortality and treatment outcome (WEISDORF et al., 1987). A corollary finding in many of the randomized clinical trials was that infectious morbidity was greater in the fed group due to poor management. Excess carbohydrate calories that produce significant hypoglycemia or excess parenteral fat administered too rapidly have been shown to diminish immune function. These findings emphasize that nutritional support therapies must be provided in an expert manner with attention to an appropriate P/E ratio. This becomes critical with parenteral alimentation. Nutritional support has the potential to cause complications and worsen outcomes if not done well.
Radiation therapy is usually longer than chemotherapy and can lead to significant anorexia. Nutritional support to the malnourished may be more likely to improve outcome when intense radiotherapy courses are provided that include the abdomen in the radiated field. However, there are insufficient data to recommend routine adjuvant nutritional support during radiotherapy.
With gastrointestinal cancer, where local obstructive symptoms may commonly be a cause of malnutrition (i.e., cancer of the esophagus), nutritional support can replete lean tissue and will improve nutritional status prior to the planned stress of major surgery as well as improve outcome (MULLER et al., 1982).
When
malnutrition develops from the metabolic response to injury or
inflammation as seen in trauma, infection, inflammatory disease,
and cancer, nutritional therapy adequate in terms of protein and
energy may reduce the rate of tissue loss. However, it will not
replete lean tissue during the acute phase, although fat gain can
be achieved (STREAT et al., 1987; NIXON et al., 1981).
For recovery from most
infections, P/E ratios in the range of those proposed to allow
for catch-up growth of severely malnourished individuals should
be adequate. For chronic infections there is so much variation
with the nature and duration of the disease that no
generalizations can be made. In the absence of other data, the
same P/E ratios as for recovery from acute infections should be
followed in dealing with recovery from chronic infections
depending on the degree of depletion and the rate of recovery.
There is no evidence of an increase in the desirable P/E ratio in
the treatment of cancer, except when recovery from depletion is
occuring as the result of remission or of successful therapy.
1. Because infections
always result in the depletion of lean body mass, it is important
that the diet be adequate in protein and energy during the
recovery period for the repletion of adults and for both the
repletion and catch-up growth of children.
2. An adequate diet should continue to be fed during diarrhea. After an acute diarrhea! episode, additional food with an increased protein content should be provided for repletion and catch-up growth of children and for the restoration of the lean body mass of adults.
3. During persistent diarrhea, patients should receive as much nourishing food as they can tolerate. If depletion becomes clinically important, this may need to be supplemented by parenteral feeding.
4. The long-term approach to the prevention of environmental enteropathy is environmental sanitation and hygiene to ensure safe water and food supplies. Until the condition can be prevented, its adverse effect on the absorption of nutrients must be taken into consideration, given the wide prevalence but modest severity of malabsorption in the individual case. An additional allowance of 10% is likely to be sufficient to compensate for the effect of this condition on intestinal function.
5. The effects of intestinal parasites on the absorption of protein, fat and other nutrients depend on the kind of parasite and the severity of the infection. Severe parasitism will continue to impair absorption until treated.