J.M. KINNEY* and D.H. ELWYN
* 8 Harvard Lane, Hastings-on-Hudson, NY 10706, U.S.A.
Abstract
1. Introduction
2. History 1900-1960
3. Indirect calorimetry and N balance in surgical patients
4. Nitrogen balance: The role of energy balance and N intake
5. Summary
References
The interrelationships
of protein and energy metabolism have been established for normal
adult individuals, in part by analyzing the dietary intake which
supports normal life without a change in body weight. Since the
same approach is not possible in ill and injured patients,
understanding of this topic has developed slowly and still
remains very incomplete. An important stimulus to further study
has been the need to develop better guidelines for the
administration of intravenous or enteral nutrition to such
patients.
Studies of energy and protein metabolism in the acutely ill during the first half of this century were characterized by measurements of nitrogen excretion and hence nitrogen balance while energy expenditure was estimated but seldom measured. The belief that injury and sepsis produced extreme hypermetabolism, led to excessive energy intakes which sometimes contributed to the stress response.
The
combined measurements of resting energy expenditure and nitrogen
balance have allowed analysis of this relationship before and
after aggressive nutritional support. The differences between the
responses to nutrition of the acutely catabolic patient and the
chronically depleted patient are emphasized when the energy
variable is considered as energy balance rather than the
conventional energy intake.
The metabolic and
nutritional interrelationships between energy and protein have
received much attention over the past fifty years, yet they
remain only partially understood. The requirements of normal
subjects for food energy and for protein have been reviewed
(PELLET, 1990a; b) in the light of recent data. Food consumption
data for the United States indicate that approximately 16% of the
total food energy is derived from protein (USDA Consumer
Nutrition Division, 1983). Despite wide variations in activity
and energy intake, this ratio is reported to be similar for both
sexes and in all age groups.
At the
present time, there is no clearly established ratio for the
optimum proportion of protein to energy intake in acutely ill or
injured patients. This situation is the result of many factors,
including inadequate knowledge of how acute catabolic conditions
influence the relationship between protein and energy metabolism,
and how this relationship changes as the acute catabolic phase
passes into the subsequent anabolic phase. Furthermore, there is
a serious lack of information concerning how the loss of body
mass, particularly of body protein, is related to the loss of
function of organs and tissues, and how promptly this functional
loss is improved with nutritional support. Ideas of
hypermetabolism and its relation to nitrogen loss evolved before
measurements were carried out during the early use of nutritional
support. This paper reviews selected parts of this history and
presents data from one laboratory regarding the influence of
nutritional support on protein and energy balance.
During the first half of
this century, nitrogen excretion and nitrogen balance were
reported in various acute clinical conditions. This was in
contrast to energy expenditure which was estimated more often
than it was measured. An exception was the extensive research of
DuBois and colleagues (DuBOIS, 1924) who studied the increase in
the basal metabolic rate (BMR) associated with various medical
conditions including fever. These studies indicated that the
basal metabolic rate was increased 13% for each degree centigrade
of fever. Examination of the data reveals that essentially all of
the acutely ill patients with typhoid fever had BMRs higher than
predicted while the patients with chronic pulmonary tuberculosis
had BMRs lower than predicted. Coleman and other associates of
DuBois published articles which mentioned the difficulty of
providing enough protein intake to offset the large nitrogen
excretion rates in the acute typhoid patients.
Focus on the metabolism of injury began with the studies of healthy males who were in the catabolic phase after having undergone long bone fractures (CUTHBERTSON, 1930). The data revealed a parallel increase in the BMR and the daily nitrogen excretion which lasted for 10 to 14 days following the fracture. The nitrogen loss was shown to correlate with the loss of other intracellular constituents, presumably from skeletal muscle. A negative nitrogen balance became the hallmark of injury, yet the parallel behavior of the BMR and the nitrogen excretion received little attention.
It became apparent to Cuthbertson, at the outset of World War II, that confusion existed among physicians caring for combat casualties, concerning the shock response to injury in contrast to the metabolic changes which followed resuscitation. He emphasized that there was an early 'ebb' or shock phase followed by a 'flow' or catabolic phase (CUTHBERTSON, 1942). The early phase was commonly considered to be one of hypotension while a negative nitrogen balance characterized the later phase. It is of interest that Cuthbertson emphasized the distinction between these phases on the basis of energy expenditure. He felt that the shock phase was a condition of ebbing energy expenditure, whereas the flow phase referred to an increased energy expenditure that resembled inflammation in certain respects.
Making a distinction between the ebb and the flow phase is important for the management of the circulation and vital organ function. However, with the later development of nutritional management by other investigators, it became important to separate the catabolic or 'flow' phase from the subsequent anabolic phase, although the turning point from one to the other is often difficult to define and determine. Physicians caring for acutely ill or injured patients have long known the clinical features of the 'turning point' when patients begin to regain their appetite, interest in their appearance and a desire to relate to other people. It is obvious that the majority of patients requiring intensive care will be in the 'flow' or catabolic phase with nutritional objectives which differ from those of the subsequent anabolic phase.
The period of 1940 onward was associated with a growing concern over the potential seriousness of weight loss in the acutely ill patient. Physicians associated weight loss with the 'toxic destruction of protein' in acute pneumonia and other fevers. Surgeons worried about operating on the seriously depleted patient, and the effect of depletion on wound healing. A specialized protein hydrolyzate was administered intravenously to patients in an effort to offset the nitrogen loss (ELMAN, 1937). This was not able to reduce the elevated nitrogen excretion of such patients, presumably because they were not receiving an adequate supply of energy. Glucose given by peripheral vein could supply only limited calories, and no intravenous fat preparation was available.
The post-injury loss of nitrogen was thought by some to be the result of unusually large increases in resting energy expenditure. Such estimates of increased energy demand might be so large (up to 2.5 times normal or higher) that they outstripped the ability of fat mobilization to provide sufficient energy; therefore protein was broken down to help provide two-carbon fragments for tissue fuel. This mistaken idea was not corrected promptly, since actual measurements of energy expenditure were difficult to perform under clinical circumstances.
SOROFF et al., on 1961 reported a study of convalescent burn patients at the Brooke Army Hospital, where the daily energy intake by mouth and tube feeding was fixed at 6400 kcal per day, reflecting the widespread belief that extreme hypermetabolism was present. The nitrogen balance above and below equilibrium intake was followed at four points in the convalescence, and a line of similar slope was shown to be present as the value for nitrogen equilibrium moved from the peak value of early convalescence back toward normal. Thus, when total parenteral nutrition containing lipid was introduced in Sweden in the early 1960's (WRETLIND, 1972) and high-carbohydrate hyperalimentation in the late 1960's in the US (DUDRICK, WILMORE and VARS, 1968), many physicians were convinced that very large energy intakes were needed to obtain any benefit.
Since
physicians were not accustomed to utilizing measurements of
energy expenditure, many thought that the average resting value
for normal subjects was closer to 2000-2500 kcal/d rather than
the actual value of roughly 1500 kcal/d. Physicians starting with
a falsely high estimate of normal calorie needs, would then
multiply this figure by a factor of 2 or 2.5 to calculate the
energy needs for presumed energy equilibrium. Since further
calories were needed to produce a positive energy balance, it was
not surprising that certain critically ill patients were often
receiving energy intakes of 5000 to 7500 kcal.
A four-bed research
intensive care unit was constructed on the surgical service of
the Columbia-Presbyterian Medical Center in New York City in
1964. Each patient room was designed with built-in equipment for
conducting continuous measurements of gas exchange over periods
of 30 minutes to 3 hours using a head canopy of special design
(KINNEY et al., 1964). The nursing staff, dieticians and
supporting laboratories were trained to perform metabolic balance
studies with particular emphasis on nitrogen balance. Patients
with various acute surgical conditions and their resultant
depletion were studied (KINNEY, 1970).
The resting energy expenditure (REE) of each patient was calculated from three to five 30-minute measurements of gas exchange during each waking day. The total energy expenditure was estimated on the assumption that physical activity contributed less than 15% in ambulatory hospitalized patients, and less than 5% in patients who were bedridden (KINNEY et al., 1968). Therefore, the daily energy requirement of such patients was predominantly related to the REE. Since many of the patients were receiving intravenous nutrition throughout much or all of each 24 hours, the value of the thermic effect of food was included in the REE.
There was considerable surprise to learn that, after major surgical operations, the REE varied from no change to increases of less than 10%. Convalescence from multiple injury, particularly if fractures of the extremities or pelvis were involved, was associated with increases in REE of 10 to 25% which lasted for 2 to 3 weeks in parallel with an increase in nitrogen excretion. The presence of fever with bacteremia was found to increase the REE approximately 13% for each degree centigrade above the normal temperature, just as had been demonstrated by DuBois. However, if the infection involved an extensive inflammatory response, such as generalized peritonitis or empyema, the REE might be increased from 30 to 50% with correspondingly high nitrogen excretion.
Extensive third-degree burns were found to have the largest increases, ranging from 40 to 100% above the predicted normal. Burned patients were commonly treated during the 1960's with open exposure in unheated environments while applying surface antibacterial ointments for periods of many weeks. The radiative as well as the evaporative cooling of these patients was increased. The severe hypermetabolism in such patients appeared to be some combination of obligatory increases in heat loss and increased heat production of internal organs as a result of the strong catabolic stimuli.
The hospital patient with advanced cachexia was found to have a decrease in REE of as much as 35 or 40% below predicted normal values. It appeared that the REE of every patient represented a balance between increases due to catabolic influences and decreases due to whatever tissue depletion had occurred.
There were no commercial devices in 1980 which were designed to be rolled to the bedside for measuring gas exchange. The decade of the 1980's produced a number of such devices, followed by a growing number of reports in the literature of the REE in various clinical conditions. The greatest change from the level of the REE reported in the 1960's was for patients with a major burn. There have been major advances in burn care over the past 20 years with a corresponding increase in the survival rate. Resuscitation is now usually prompt, pulmonary injury is recognized and treated, the environment is warmed, excision of the burn surface is started soon after resuscitation and closed dressings minimize evaporative cooling. With such modern techniques, the burned patient seldom demonstrates elevations of REE greater than 60% above normal, and lower values are common.
The hypometabolic end of the clinical spectrum due to tissue depletion is also less marked since malnourished patients usually receive aggressive nutritional support before reaching the stage of advanced cachexia. Therefore, the range of clinical REE of the 1960's which extended from 100% above normal to 40% below normal, has now been reduced to a range which approximates plus 60% to minus 20%. While this is a significant reduction, the REE should be measured if possible, since it is difficult to estimate correctly in ill patients and remains a 'catabolic marker' for other pathophysiological changes, particularly abnormal proteolysis and ureagenesis.
In partial
starvation, uncomplicated by disease, nitrogen losses are
commonly in the range of 7 to 12 g N/d. Most patients after a
large operation, sepsis or long bone fractures will have losses
in the range of 12 to 20 g/d. The largest losses are seen in the
young, heavily muscled male, while the smallest losses tend to be
seen in the female, the elderly and the depleted. The most
extreme nitrogen losses in severe catabolic conditions may reach
as much as 30 g/d (KINNEY and ELWYN, 1983). The extent of the
nitrogen loss depends upon the severity of the illness or injury,
the extent of immobilization, and the presence of any prior
malnutrition.
4.1. Normal subjects
4.2. Depleted patients
4.3. Injured patients
It is generally recognized that nitrogen balance is a function of both N and energy intake when all other nutrients are supplied in adequate amounts (MUNRO, 1965). In other words, unless one or the other has become limiting, an increase in either N intake or energy intake will cause an increase in N balance. In comparing different individuals, there is an inherent problem in using energy intake as such, since energy requirements will vary. At an intake of 2200 kcal, one subject might be in positive energy balance and, therefore, in positive N balance. Another individual, at the same energy intake, might be in negative energy balance and so in negative N balance. This problem can be overcome by using energy balance as the appropriate parameter influencing N balance, since this includes both energy intake and energy requirements.
Use of
energy balance instead of energy intake is supported by a study
of 10 depleted patients studied by indirect calorimetry and
nitrogen balance while receiving different levels of intravenous
nutrition (ELWYN et al., 1979). When N balance was
regressed against energy intake, the correlation coefficient (r)
was 0.72 (our calculation). The corresponding value for r2
was 0.52, indicating that 52% of the variability in N balance
could be explained by changes in energy intake. When N balance
was regressed against energy balance, r was 0.87 and r2
was 0.76 indicating that 76% of the variability in N balance
could be explained by changes in energy balance.
In normal adult subjects at
zero energy balance, a change in N intake will cause a transient
change in N balance; however, after three to four days, N balance
returns to zero at any intake which is above minimum
requirements. This must be so since at zero energy balance, when
there is neither loss or gain of weight and if exercise patterns
are constant from day to day, neither loss nor gain of N can
continue indefinitely. With a positive energy balance, normal
adults will gain weight in the approximate proportions of 1 part
body cell mass to 2 parts of fat, and with negative energy
balances, not too far from zero, weight will be lost in about the
same proportions (BURSZTEIN et al., 1989). Under these
conditions it is probable that the intake of N will affect the
rate of gain or loss of N. With energy intakes ranging from zero
to one-half of requirements, about 2 parts of body cell mass are
lost for I part of fat (BURSZTEIN et al., 1989). The ratio
of loss of body cell mass to fat in patients after elective
operation is about 2:1, and after severe trauma it is about 4:1
(KINNEY et al., 1970).
Quantitative relationships
between energy balance and nitrogen balance at different intakes
of N are shown in Figure 1. This scheme is based on
experimental studies in which N intake was changed while energy
balance was held constant or energy balance was changed while N
intake was kept constant. Nevertheless, the results of many
studies, in which both parameters were changed simultaneously,
are in good agreement (ELWYN et al., 1979) although they
could not be used to derive the relationship. The data used were
steady-state values taken from the last 2 to 4 days of 6- or
8-day study periods.
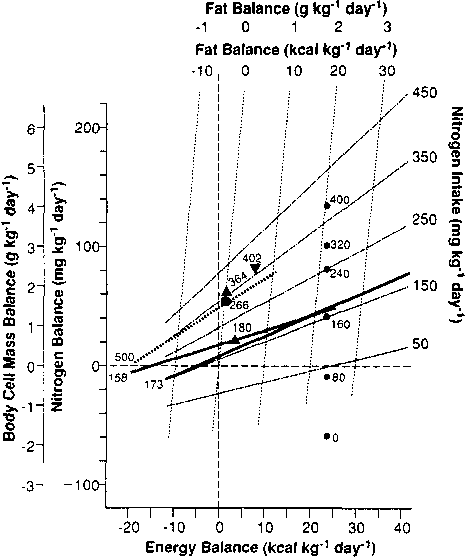
The heavy lines and solid symbols represent experimental data: the associated numbers are for the particular N intake. The positions of the nitrogen intake lines are based on data of PLOUGH et al. (1956); SHAW et al. (1983); RUDMAN et al. (1975); CHIKENJI et al. (1987); and FORSE et al. (1990). Detailed derivation is described in SHAW et al., (1983). Reproduced from ELYWN (1991) with permission of J.B. Lippincott.
Figure 1 shows that, in the malnourished adult, hospitalized patient, increasing either energy balance or N intake increases N balance. Unlike the normal adult situation, a markedly positive N balance may be obtained in such patients at zero or even a negative energy balance. The effects of increasing N and energy intake are synergistic; at an energy balance of 30 kcal/kg/d, 40% of an increase in N intake is retained, while at zero energy balance only 20% is retained. The requirement of 120 mg N/kg/d to maintain zero N balance at zero energy balance in such patients, is substantially higher than the 80 mg N/kg/d required for normal subjects, indicating that these patients have considerable underlying illness. It may be noted that, because they are an ill population, standard errors for N balance are much higher than normal ranging from 2 to 3 g per day per subject or 30-50 mg N/kg/d.
Fat balance
is also included in the chart since, at steady state, when no
change in glycogen occurs, fat balance equals energy balance
minus N balance when all are expressed in calories. Since the
body cell mass is about 30 times its N content, the chart can be
used to estimate the proportions of body cell mass and fat that
will be restored on a given diet. At an energy balance of 12
kcal/kg/d and a N intake of 400 mg/kg/d, 3 g of body cell mass
will be restored for each gram of fat. This would be suitable for
patients with rapid prior weight loss due to injury. At an energy
balance of 10 kcal/kg and a N intake of 150 mg/kg/d, 0.5 g of
body cell mass would be restored for each gram of fat. This would
be suitable for patients who had had slow, chronic weight loss
due to anorexia nervosa or chronic obstructive pulmonary disease.
The effect of injury to
well-nourished subjects is to increase tissue losses resulting in
negative N balance. Initially this effect cannot be overcome with
normal diets. The extent and duration of negative N balance is
proportional to the severity of the injury, but even with an
elective operation, such as cholecystectomy, about 15 g of N loss
over the first 3 days seems obligatory, despite energy intakes in
excess of expenditure with adequate protein (ROWLANDS et al., 1977).
Nitrogen losses with severe injury or burns may approach 30 g per
day with 5% dextrose infusions. Provision of energy at 50% above
measured expenditure together with 14 g N per day will reduce
losses to about 5 g N per day, an acceptable risk (GOLDSTEIN and
ELWYN, 1989). Further increase in N intake has little effect.
Large increases in energy intake will probably reduce N losses,
but only at the cost of significant fat deposition.
Malnourished
patients who are injured or become further depleted will respond
more favorably to increases in N and energy intake than do
well-nourished individuals (ELWYN, in press).
The interrelationships of
protein and energy for the normal subjects are far better
understood than for the injured, septic or depleted patient. This
arises in part because the nutrient requirements of the normal
subject have been established in terms of averages from large
population groups, while the acutely ill or depleted patient is
treated as a single individual with many special considerations
which may modify the conventional nutritional intake at that
time.
A knowledge of resting energy expenditure and nitrogen excretion is important to characterize the existing metabolic state, as well as to establish the daily balance of calories and nitrogen which is desirable. The metabolic state of a patient changes during convalescence from acute catabolism in the previously well-nourished patient to a state of depletion which is usually associated with an anabolic state.
The
response of the nitrogen balance to the intake of calories
and nitrogen is partially known for the hospitalized patient.
This approach is extended in this paper to consider the energy
balance as well as the nitrogen intake. The difference in the
nutritional response of the acutely ill and the chronically
depleted patient to nutritional support becomes more evident from
this conceptual approach.
BURSZTEIN, S., ELWYN, D.H.,
ASKANAZI, J., KINNEY, J.M.: Energy Expenditure, Indirect
Calorimetry and Nutrition. Williams & Wilkins, Baltimore, MD,
1989.
CHIKENJI, T., ELWYN, D.H., GIL, K.M., DANFORTH, E., Jr., ASKANAZI, J., KINNEY, J.M.: Effect of increasing glucose intake on nitrogen balance and energy expenditure in malnourished adult patients receiving parenteral nutrition. Clin. Sci., 72, 489-501 (1987).
CUTHBERTSON, D.P.: The disturbance of metabolism produced by bony and non-bony injury, with notes on certain abnormal conditions of bone. Biochem. J., 24, 1244-1249 (1930).
CUTHBERTSON, D.P.: Post-shock metabolic response. Lancet, 1, 433-436 (1942).
DuBOIS, E.F.: Basal Metabolism in Health and Disease. Lea & Febiger, Philadelphia, PA, 1924.
DUDRICK, S.J., WILMORE, D.W., VARS, H.M. et al., Long-term parenteral nutrition with growth, development and nitrogen balance. Surgery, 64, 134-142 (1968).
ELMAN, R.: Amino acid content of blood following intravenous injection of hydrolyzed casein. Proc. Soc. Exp. Biol. Med., 37, 437-443 (1937).
ELWYN, D.H., GUMP, F.E., MUNRO, H.N., ILES, M., KINNEY, J.M.: Changes in nitrogen balance of depleted patients with infusions of glucose. Am. J. Clin. Nutr., 31, 1597-1611 (1979).
ELWYN, D.H.: Energy and nitrogen metabolism: scientific basis for clinical practice. In: Problems in General Surgery, Vol. 8, No. 1, pp. 14-22. Lippincott, Philadelphia, PA, 1991.
FORSE, R.A., ELWYN, D.H., ASKANAZI, J., ILES, M., SCHWARZ, Y., KINNEY, J.M.: Effects of glucose on nitrogen balance during high nitrogen intake in malnourished patients. Clin. Sci., 78, 273-281 (1990).
GOLDSTEIN, S.A., ELWYN, D.H.: The effects of injury and sepsis on fuel utilization. Annul Rev. Nutr., 9, 445-473 (1989).
KINNEY, J.M., MORGAN, A.P., DOMINGUES, F.J., GILDNER, K.J.: A method for continuous measurement of gas exchange and expired radioactivity in acutely ill patients. Metabolism, 13, 205-211 (1964).
KINNEY, J.M., LONG, C.L., GUMP, F.E., DUKE, J.H., Jr.: Tissue composition of weight loss in surgical patients. l. Elective operation. Ann. Surg., 168, 459-464 (1968).
KINNEY, J.M., DUKE, J.M., Jr., LONG, C.L., GUMP, F.E.: Tissue fuel and weight loss after injury. J. Clin. Path., 23 (Suppl. 4), 65-72 (1970).
KINNEY, J.M., ELWYN, D.H.: Protein metabolism and injury. Annul Rev. Nutr., 3, 433-456 (1983).
MUNRO, H.N.: General aspects of the regulation of protein metabolism by diet and hormones. In: Mammalian Protein Metabolism, Vol. l, pp. 382-482. H.N. MUNRO, J.B. ALLISON (Eds.). Academic Press, New York, NY, 1965.
PELLET, P.L.: Food energy requirements in humans. Am. J. Clin. Nutr., 51, 711-722 (1990a).
PELLET, P.L.: Protein requirements in humans. Am. J. Clin. Nutr., 51, 723-737 (1990b).
PLOUGH, I.C., IBER, F.L., SHIPMAN, M.E., CHALMERS, T.: The effects of supplementary calories on nitrogen storage at high intakes of protein in patients with chronic liver disease. Am. J. Clin. Nutr., 4, 224-230 (1956).
ROWLANDS, B.J., GIDDINGS, A.E.B., JOHNSTON, A.O.B., HINDMARSH, J.T., CLARK, R.G.: Nitrogen-sparing effect of different feeding regimens for patients after operation. Br. J. Anaesth., 49, 781-787 (1977).
RUDMAN, D., MILLIKAN, W.J., RICHARDSON, T.J., BIXLER, T.J., STACKHOUSE, W.J., McGARRITY, W.C.: Elemental balances during intravenous hyperalimentation of underweight adult subjects. J. Clin. Invest., 55, 94-104 (1975).
SHAW, S.N., ELWYN, D.H., ASKANAZI, I, ILES, M., SCHWARZ, Y., KINNEY, J.M.: Effects of increasing nitrogen intake on nitrogen balance and energy expenditure in nutritionally depleted adult patients receiving parenteral nutrition. Am. J. Clin. Nutr., 37, 930-940 (1983).
SOROFF, H.S., PEARSON, E., ARTZ, C.P.: An estimation of the nitrogen requirements for equilibrium in burned patients. Surg. Gynecol. Obstet., 112, 150-172 (1961).
WRETLIND, A.: Complete intravenous nutrition: theoretical and experimental background. Nutr. Metab. (Suppl.), 14, 1 (1972).