P.S. SHETTY*
* ICMR, Nutrition Research Centre, Department of Physiology, St. John's Medical College, Bangalore 560034, India.
1. Respiratory quotients in semi-starvation
2. Respiratory quotients in experimental semi-starvation
3. Respiratory quotients and substrate oxidation rates in chronically energy deficient subjects
4. Substrate oxidation rates during dietary thermogenesis in chronic energy deficiency
5. Effects of refeeding or supplementation on respiratory quotients and substrate oxidation rates of CED subjects
Acknowledgements
References
Nutrient
interactions are of considerable interest in the field of energy
metabolism. Jéquier (this volume) has outlined the interaction
between ingested nutrients and their influences on nutrient
metabolism and substrate utilisation in vivo. He has
discussed the effects of dietary carbohydrate intakes on fat
oxidation and presented data on the effects of varying
proportions of energy and protein intakes on nitrogen metabolism,
which is the main theme of this meeting. He has summarised the
available data in normal subjects and in hospitalised patients on
total parenteral nutrition. In this discussion paper, I shall
attempt to highlight the differences between normally nourished
and chronically energy deficient individuals in substrate
oxidation, nutrient utilisation and the associated thermic
effect. Since respiratory quotients (RQs) are a proxy for the
substrates being oxidised in the body, the literature pertaining
to semi-starvation is reviewed largely with reference to the RQs
observed in these conditions.
One of the most valuable
and detailed scientific reports on the effects of famine on man
is based on observations made by Jewish physicians in the Warsaw
ghetto (FLIEDERBAUM, 1979). It includes metabolic studies carried
out on 70 individuals with protracted, severe undernutrition. The
fasting, non-protein RQs recorded by the Jewish physicians were
unusually high, with values between 0.95 to 0.98 in the majority
of cases. This finding led them to conclude that the higher than
normal RQ and the concomitant reduced nitrogen excretion in the
urine were strongly suggestive of the exclusive utilisation of
carbohydrate as metabolic fuel by these severely undernourished
individuals. It is not clear from these accounts, whether the
subjects maintained stable weight at the time and what their
dietary intakes were like. The Jewish physicians also observed in
these severely under nourished individuals that supplementation
of 300 g of cane sugar daily for a week, in addition to the
regular hospital diet, resulted in a rise in the fasting
protein-free RQ. A similar rise in RQ was also observed within
two hours following an oral sucrose load. These observations led
them to conclude that patients with famine starvation or
'hunger-disease', as they preferred to call it, had a high
fasting RQ, demonstrating a physiological ability of these
individuals to burn endogenous carbohydrate. The further rise in
RQ after an oral sucrose load implied that they retained the
ability to rapidly burn exogenously administered sugars. The
Jewish physicians also made the observation that, in terminal
cases of hunger-disease, with losses of body weight exceeding
50%, the RQ was between 0.72 to 0.74. It is not clear whether
these individuals with terminal undernutrition were losing weight
at the time of the BMR measurements. It appears that the
relatively high RQs of these patients fell to low levels in the
terminal stages of severe weight loss, just prior to their death.
Two of the major systematic
studies of experimental semi-starvation in man are the
"Carnegie experiment", conducted by Benedict and his
colleagues in the Carnegie Nutrition Laboratory in 1917-18
(BENEDICT et al., 1919), and the "Minnesota
experiment", conducted by Keys and his co-workers in 1945 at
the University of Minnesota (KEYS, 1954). The fasting RQs
recorded during semi-starvation in the Carnegie experiment were
highly variable. The average basal RQ of 0.8, recorded in the
volunteers during the control period, was frequently found to be
elevated following experimental semi-starvation; in others it
remained unchanged, and in a few instances it was below 0.73.
During the
semi-starvation phase of the Minnesota study, unfortunately no
systematic measurements of RQ were made in the basal, fasted and
resting state. Some measurements that were made near the end of
the semi-starvation phase showed a mean RQ of 0.84 (range from
0.79 to 0.89). During nutritional rehabilitation, when the food
intake and weight gain were highest, the mean basal RQ appeared
to be marginally higher at 0.85 (range 0.83 to 0.88) in the few
individuals who were studied. However, exercise RQs were
determined during the various phases of the Minnesota study. The
mean exercise RQs increased throughout the period of starvation
and through half the period of rehabilitation and then decreased
slightly.
Several reports on BMRs of
Indians have indicated that their RQs vary over a wide range and
that they generally tend to be higher than 0.82, a value which is
frequently quoted in the Western literature as a normal average (Table
1). SHIV KUMAR et al. (1961), who studied 339
apparently normal, healthy subjects aged between 18 to 80 years,
noted that the RQs of these subjects varied from 0.70 to 0.97,
with a mean value of 0.83 (SEM ± 0.003) and a coefficient of
variation (CV) of 7.1%. These authors stated in their paper that,
since the publication of the large study on BMRs of Indians with
concurrent measurements of RQ by SEN and BANERJEE (1958),
evidence has been accumulating that the average RQ of Indians is
somewhat higher than 0.82. SHIV KUMAR and others (1961) also made
the suggestion that the wide normal range of basal RQs in their
study (ranging from 0.70 to 0.97) deserved greater attention and
emphasis than the slight differences seen in the means as
compared to the mean RQs reported in Western subjects. It is
assumed that all these observations were made in apparently
healthy individuals who maintained stable weight.
The large range of RQ values seen in these studies from India may be attributed to the varying nutritional status of these apparently normal adults who were weight stable. It is not unlikely that a fair proportion of these subjects had low body weights and low body mass indices (BMIs), and that a proportion of the apparently healthy individuals in this reasonably large sample were weight stable but chronically energy deficient. Table 2 summarises the RQs of undernourished or CED subjects from the same geographical region. It clearly shows that individuals of poor nutritional status have a higher basal fasting RQ than well-nourished controls from the same region. The wide range of RQ values reported in Table 1 may thus represent contributions from undernourished subjects, present in the large samples of individuals whose BMRs were reported.
Table 1. Respiratory quotients of apparently healthy Indians reported in the literature
Number of subjects
|
Respiratory quotient |
Reference |
|
Mean |
Range |
||
18 |
0.84 |
0.71-0.99 |
MUKERJEE and GUPTA, 1931 |
9 |
0.81 |
0.72-0.87 |
AHMED and Roy, 1938 |
138 |
0.84 |
0.71-0.98 |
SEN and BANERJEE, 1958 |
99* |
0.84 |
0.73-0.98 |
SEN and BANERJEE, 1958 |
339 |
0.83 |
0.70-0.97 |
SHIV KUMAR et al., 1961 |
* Female subjects.
Table 2. Respiratory quotients of chronically energy deficient or undernourished Indians
Undernourished |
Well-nourished |
Reference |
0.94 |
0.84 |
RAMANAMURTHI et al., 1962 |
0.94 (0.02) |
0.77 (0-03) |
SHETTY, 1984 |
0.94 (0.02) |
0.79 (0-05) |
SOARES et al., 1991 |
0.90 (0.07) |
0.87 (0.05)1 |
SOARES and SHETTY, 1991 |
0.93 (0.08) |
0.84 (0.06)2 |
SOARES and SHETTY, 1991 |
0.93 (0.03) |
0.81 (0.01) |
PIERS et al., 1992b |
1 Rural population.
2 Urban population.
Figures in parenthesis are standard errors.
Table 3. Comparisons of respiratory quotients (RQ) with food quotients (FQ) in chronically energy deficient (CED) subjects
|
Controls |
CED Subjects |
||
RQ |
FQ |
RQ |
FQ |
|
Fasting RQ vs FQ (24-h recall) |
0.86 |
0.84 |
0.95 |
0.94 |
Fasting RQ vs FQ (weighed) |
0.85 |
0.81* |
0.93 |
0.88* |
Fasting RQ vs FQ (weighed)+ |
0.83 |
0.82 |
0.93 |
0.90* |
* Statistically significant.
+ During 36 hours of calorimetry.
Table 4. Respiratory quotients and substrate oxidation rates of chronically energy deficient (CED) subjects in the post-absorptive, fasted state
|
Respiratory quotient |
Substrate oxidation
rates |
|||||
g/h |
kJ/kg/h |
||||||
C |
F |
P |
C |
F |
P |
||
Well-nourished |
0.81 |
5.0 |
3.8 |
2.9 |
1.3 |
2.1 |
0.7 |
CED subjects |
0.93* |
8.1* |
1.1* |
2.4 |
3.1* |
0.9* |
0.9 |
C = carbohydrate |
F = fat |
P = protein |
|||||
* Statistically significant differences by analysis of variance.
From PIERS et al., 1992b.
The higher RQs of Indians have generally been attributed to their diets containing a high proportion of carbohydrates. This seems a reasonable assumption. Since mean RQs of free-living subjects are not readily and continuously available, BLACK and her colleagues (1986) suggested a different approach to predict the RQ from the macronutrient content of the diet. This was termed the food quotient (FQ). Under conditions of energy balance and weight maintenance, the FQ must equal RQ, and the RQ should reflect the macronutrient composition of the diet.
Comparisons of FQs, made in a number of well-nourished and CED subjects, and calculated according to the methods suggested by BLACK et al., (1986), were compared with the 12-14 hour postabsorptive, early morning RQs obtained during the measurement of a BMR. No differences were seen between the FQs based on dietary recall and post-absorptive, fasting RQs in either group of subjects studied (Table 3). As noted above, the fasting RQs of the CED subjects were significantly higher than those of the well-nourished. Their FQs corroborated this, since the antecedent habitual diets of these individuals were high in carbohydrate content. The lower FQs in the well-nourished controls were accounted for by a higher fat intake than in the CED subjects, despite the carbohydrate intakes in both groups being higher than the usual Western diets. However, comparisons of FQs from weighed intakes over several days, with the RQs of the same subjects obtained during a standard BMR measurement, showed that both had FQs that were significantly lower than RQs.
Fasting RQs obtained during a 36-hour cycle in an indirect calorimeter were also computed in both well-nourished controls and undernourished CED subjects while in the whole-body calorimeter. Differences between RQs and FQs were seen only in the CED subjects, with RQs being higher than FQs. The FQs were obtained from the composition of the actual food provided to the subjects during 36-hour calorimetry runs. That food was different from their habitual diets in terms of the macronutrient composition. It seems, however, that the macronutrient composition of the diet ingested, as indicated by the mean FQ of the day, is not truly reflected in the fasting RQ of the same day. Since the individuals were maintaining stable body weights and therefore were unlikely to be in a state of energy imbalance, RQs should equal FQs. The higher fasting RQ may be compensated for by FQ > RQ at other times of the day. Records of the 24-hour RQs in these CED subjects do show that the post-exercise RQs are lower than the FQs.
When substrate oxidation rates were calculated during the postabsorptive, fasted state in these CED subjects, using indirect calorimetry and urinary N excretion (without correcting for changes in the blood urea pool), the CED subjects had significantly higher rates of carbohydrate oxidation and lower rates of fat oxidation in the fasted state than the well-nourished controls (Table 4). No differences were seen in the rates of protein oxidation; an observation that is in keeping with the evidence of similar rates of protein turnover in CED and well-nourished subjects (SOARES et al., 1991). It would appear that the CED subjects have a higher RQ largely due to their selective use of carbohydrate as fuel even in the post-absorptive, fasted state.
It is important to recognise that, contrary to general belief, fat is not necessarily the predominant substrate in the post-absorptive, fasted state and is certainly not the preferred substrate in the chronically undernourished. However, the selective utilisation of carbohydrate illustrates how closely carbohydrate oxidation is adjusted not only to its immediate availability, as demonstrated in well-nourished individuals (FLATT et al., 1985), but probably also relates to the antecedent habitual intakes of carbohydrate in the diets of the undernourished.
The selective use of carbohydrate as fuel has obvious metabolic advantages to the CED individual, since carbohydrate (glycogen) oxidation generates more ATP than iso-energetic amounts of fat or protein (LIVESEY, 1984; WATERLOW, 1988). Also the metabolisable energy equivalent, i.e., the energy equivalent of ATP gained (in kJ per mole ATP) is almost identical to that of fat (75.3 for glycogen oxidation via glycolysis and the citric acid cycle; ELIA and LIVESEY, 1992). It is hence not unlikely that the high fasting RQs of the CED reflect the metabolic efficiency of active tissues of these subjects.
FLATT (1987), in an elegant hypothesis, has implicated adequacy of carbohydrate stores as being central to ingestive behaviour in humans. The need to meet adequate carbohydrate intakes may result in the ingestion of more lipid in a habitually high-fat diet, which may result in positive energy balance and excess fat storage, thus predisposing to obesity. A corollary to the main hypothesis is that, for the purposes of long-term equilibration and body weight stability, an increase in body fat may in turn lead to an increase in fat oxidation (SCHUTZ et al., 1992). A direct extrapolation of Flatt's hypothesis must surely imply that substrate oxidation is related to the fat mass of an individual. In broader, general terms, this must mean that the fasting RQ is related to the body composition of an individual.
Meta-analysis of several recent studies done by our group in well-nourished and CED subjects who were weight stable (means of 15 separate sets of data points), was used to look at relationships between RQ and body composition parameters (Figure 1). Body weight and FFM were negatively and linearly correlated to RQ (Body weight: r = 0.92; p < 0.001 and FFM: r = -0.92; p < 0.001) as well as fat mass expressed as a ratio of active tissue mass, i.e., Fat/FFM. The latter relationships were also negative and linear (Fat mass: r = -0.87; p<0.001 and Fat/FFM: r = -0.83; p<0.001). The RQ of an individual seems to reflect his body composition and more specifically the available fat stores. The high RQs of the CED subjects may thus reflect lower than normal fat stores in the individual. FLATT (1972) has suggested that the antilipolytic effect of insulin is less effective in the presence of an increased fat mass, and an increased level of insulin is thus associated with high free fatty acid levels in obesity. Since fat oxidation has been shown to be directly related to the levels of free fatty acids (ISSEKUTZ et al., 1968; GROOP et al., 1991), it is apparent that low fasting levels of free fatty acids are likely to be associated both with the small fat mass seen in CED subjects and consequently associated with lower rates of fat oxidation. The low rates of fat oxidation in the CED will contribute to the high fasting RQs. The high RQs of the CED subjects may thus reflect both a high dietary intake of carbohydrate as well as a predominant dependence on carbohydrate as fuel and a reduced rate of fat oxidation in the presence of low levels of circulating free fatty acids associated with the low fat stores observed in these individuals.
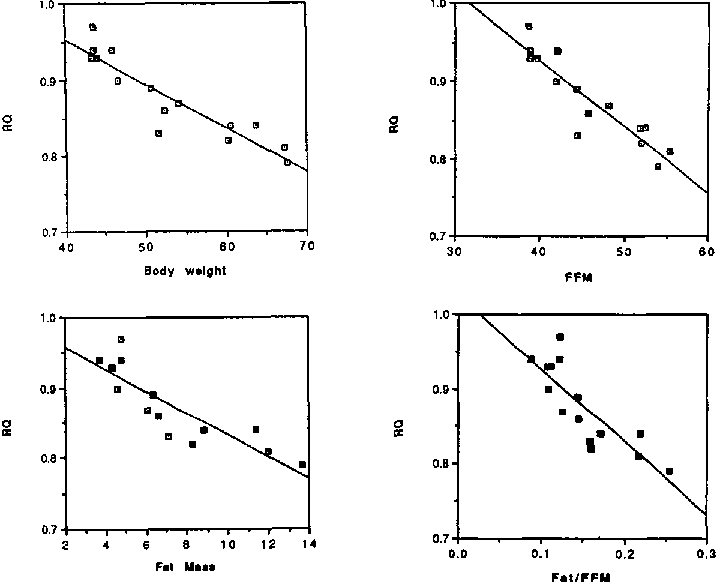
From a conceptual point of
view, there are two major components of thermogenesis which have
been designated as obligatory and facultative (LANDSBERG and
YOUNG, 1984). Obligatory thermogenesis represents the minimal
heat produced as a result of those biological processes that
maintain the organism at thermoneutral temperatures in the basal,
fasted state. BMR provides a rough estimate of obligatory
thermogenesis. Facultative thermogenesis represents heat
production in excess of that required to maintain the basal state
and is thus highly variable depending upon the needs of the
organism. The excess heat production that occurs in response to
feeding is designated diet-induced thermogenesis (DIT). The
energy cost of processing food depends on the macronutrient
composition and on metabolic rate; it is highest for protein and
lowest for fat. The thermic response to a test meal depends upon
its energy density and also its macronutrient composition. The
meal stimulus provided in studies concerned with inter-group
comparisons may be either a standard, uniform meal, or one whose
energy and/or protein content is based on the body weight, FFM or
BMR of the subject. The thermic responses of the subjects may be
expressed either as absolute values or corrected for body weight,
FFM or BMR differences of the subjects, or even expressed as a
percent of the energy density of the administered meal stimulus.
This issue remains unresolved and is further compounded by the
large variability in the responses of the same individual to the
same meal stimulus on different occasions (PIERS et al., 1992a).
DIT responses to a standard meal (600 kcal [2.5 MJ] energy, 10% protein, 15% fat), measured over a period of 6 hours, were larger in CED subjects than in well-nourished controls, both in absolute terms and when expressed as a percentage of the metabolisable energy content of the meal (PIERS et al., 1992b). The non-protein RQs and substrate oxidation rates (Table 5) were suggestive of the predominant utilisation of carbohydrate as fuel in the fed state, with little fat or protein being oxidised by the CED subjects. The increase in DIT, despite the low protein oxidation rate, may indicate an increase in protein synthesis after the meal. It is also recognised that iso-energetic amounts of carbohydrate produce a higher DIT response than fat (SCHWARTZ et al., 1985; SWAMINATHAN et al., 1985). Thus, the predominant dependence on carbohydrate as fuel, both before and after a meal, may account for the higher DIT response in the CED. DIT responses seen in the CED subjects may hence be representative of the predominant nutrient being utilised which, in turn, may depend upon the habitual dietary intake and its macronutrient composition, rather than represent variations in facultative thermogenesis.
Table 5. Non-protein respiratory quotients (NPRQ) and substrate oxidation rates (SOR) in the fasted and fed state in chronic energy deficiency
|
CONTROL SUBJECTS |
CED SUBJECTS |
||
Fasted |
Fed |
Fasted |
Fed |
|
NPQR |
0.81 |
0.88 |
0.93 |
0.97 |
SOR |
g/h |
g/h* |
g/h |
g/h* |
carbohydrate |
5.0 |
9.4 |
8.1** |
11.4** |
fat |
3.8 |
2.4 |
1.1** |
0.3** |
protein |
2.9 |
3.7 |
2.4 |
2.1** |
* Mean of 6 hours post-prandial.
** Statistically significant as compared to the control response.From PIERS et al., 1992b.