A.A. JACKSON*
* Department of Human Nutrition, University of Southampton, Bassett Crescent East, Southampton, Hampshire SO9 3TU, UK.
Abstract
1. Introduction
2. General considerations
3. The Millward model
4. Conclusions
References
The interaction between
energy and protein within the body varies with the functional
metabolic demand. The metabolic demand for energy is measured as
the flow of carbon through the body, and the main determinant of
variability within and between individuals is the level of
physical activity. The metabolic demand for protein is measured
as the flow of nitrogen through the body and the main determinant
of variability within and between individuals is the rate of
growth. Although the demands for nitrogen and carbon often move
together in the same direction, this is not necessarily so. At
marginal levels of energy intake, positive nitrogen balance may
be defended in the face of a negative energy balance.
Nitrogen
balance only represents a fraction of the intensity of the
movement of nitrogen within the body, as there are two major
internal cycles for nitrogen. The first, characterised as protein
turnover, represents the movement of nitrogen as amino acids into
and from proteins. The intensity and pattern of this movement
vary with the pattern of the metabolic demand. The second, less
clearly recognised, represents the movement of nitrogen from
amino acids into urea, and the return of the urea-N to amino acid
synthesis. The return of the urea-N requires the salvaging of
nitrogen through the metabolic activity of the colonic
microflora. Within the range of adequate protein intakes, the
production of urea is unrelated to protein intake. The
achievement of nitrogen balance appears to be dependent upon the
salvage of urea-N, implying that the activity of the colonic
microflora is an integral part of the mechanism through which
nitrogen balance is maintained ordinarily. Nitrogen, amino acids
and protein are not terms which can be used casually or
interchangeably, and the movement of nitrogen through the body
can only be measured directly with the use of nitrogen labels and
not imputed indirectly from the use of carbon-labelled amino
acids.
Millward has discussed
protein-energy interactions in the context of a model of protein
metabolism developed with Rivers, which he is in the process of
exploring in detail (MILLWARD and Rivers, 1988). The model is
valuable as an approach against which our understanding of amino
acid and protein metabolism can be assessed. It contains many of
the most important elements which need to be taken into
consideration, although some of the points require much more
refined elaboration and more careful consideration. For example,
at the present time it is not possible to quantify many of the
rates of flow within and between the different pools or
functions, and therefore it is not possible critically to
challenge the model by direct experimental exploration. I find
the concept of the anabolic drive difficult to deal with, as to
date it has not been identified with any biological reality.
For these
reasons, the model, as it stands, represents an attempt at the
description and interpretation of experimental observations which
do not appear to fit easily into prevailing concepts of amino
acid and protein metabolism. Therefore, it is important to be
sure, in trying to interpret the available data, that the control
of the experimental situation has been appropriate and allows for
the interpretations which have been made. Metabolism has to be
considered as a whole, and hence protein metabolism has to be
viewed in the context of the metabolism of other nutrients.
Comparisons can only be made legitimately, if like is being
compared with like. Energy and nutrient intake both have a major
determinant effect upon nitrogen balance and protein metabolism.
I do not intend to deal with all the points at issue, but have
selected some of the more important general points and then
considered some of the specifics of the model itself.
2.1. Functional metabolic demand
2.2. Carbon flux and nitrogen flux
2.3. Functional metabolic mass of protein
2.4. Specific limiting nutrients
2.5. Limitations imposed by protein quality
2.6. Amino acids: Essential, non-essential and conditionally essential
It is not possible to deal
with the effects of energy and protein intake upon metabolism
without a sense of the metabolic demand which the intake is
required to satisfy (JACKSON, 1985). The primary determinants of
metabolic demand can be identified either as components (ion
pumping, protein turnover, net accretion in growth) or as
processes (mechanical work, cardiovascular function, ventilation,
digestion, formation of urine, cerebration, etc.). There is
variability in metabolic demand, and the physiological factors
which introduce the largest variability within and between
individuals are physical activity for the demand for energy, and
growth for the demand for protein. In pathological situations,
inflammation increases the demand for both energy and protein.
In terms of
food intake, the primary objective appears to be to satisfy the
demand for energy with all other nutrients being consumed in
relation to nutrient density. In the context of the present
discussion, the dietary intake should legitimately be considered
to represent a component of the metabolic demand, as the evidence
is quite clear that the intake of protein presents a metabolic
demand upon energy homeostasis, and an intake of energy presents
a metabolic demand upon protein homeostasis. For any given
situation, the metabolic demand can either be not satisfied,
adequately satisfied or exceeded. Each of these situations
represents a fundamentally different metabolic state. In the
child, the functional demand includes the requirements for
growth, but, in the adult, the achievement of balance is seen as
the preferred state.
In preparation for the
FAO/WHO/UNU Expert Consultation on Energy and Protein
Requirements (1985), DANIDA supported a series of international
collaborative studies to explore the inter-relationships between
protein and energy metabolism around marginal levels of intake
(WATERLOW, 1984a). As Millward has already shown, nitrogen
balance could be maintained in children ingesting diets of poor
protein quality. One other point of importance which emerged from
these studies was the relation between energy balance and
nitrogen balance. Energy balance is a measure of carbon flux
through the body, whereas protein balance is derived from and
related to measures of nitrogen flux (WATERLOW, 1984b). Although
frequently the two processes move together in the same direction,
this is not necessarily so. In the DANIDA studies, nitrogen
balance was defended at the expense of energy balance (measured
as weight loss) at the lowest levels of energy intake (KENNEDY,
BADALOO and JACKSON, 1990). Similarly, JAHOOR et al.
(1989) have shown that, during infection or severe trauma,
gluconeogenesis and ureagenesis are not necessarily linked and
appear to be controlled as independent processes.
The meaning of the concept
of 'body protein stores' is not altogether clear to me. There is
very little evidence that protein stores exist as such, rather
there is a functional mass of protein which is formed and
maintained in relation to the metabolic demand to satisfy
specific functions or processes. The protein content of the body
is, therefore, responsive to functional demands and will be
determined both qualitatively and quantitatively by the nature
and magnitude of the functional demands. Millward suggests that
the 'body protein stores' have a set upper limit, and he implies
that this upper limit is fixed. He gives no indication of the
possible or likely determinants of the set of the upper limit,
nor what factors might influence the set. Presumably for any
given individual the 'store' cannot represent a single value, but
is an expression of the functional state at that time, as
determinded by a combination of factors which in general terms
relate to:
(a) genetic factors
(b) the metabolic demand/physiological state (e.g., pregnancy)
(c) the nature and sufficiency of other dietary nutrients.
This latter
point is related to the comment of Millward that "the more
you eat the more you need". The corollary would be "the
less you eat the less you need". It would be useful to know
the extent to which the limits of this relationship can be
defined. The ingestion and processing of food represents a
functional demand in its own right which requires both energy and
protein. Are Millward's comments likely to relate directly to the
processing activities themselves, or do they also imply changes
in other aspects of nitrogen/protein metabolism? In functional
terms, the comment has to be true, and the truth can be seen at
the simplest level as an increase or decrease in the amino acid
catabolizing enzymes and urea cycle enzymes in response to
increased or decreased dietary protein (DAS and WATERLOW, 1974).
These changes of themselves represent an increased demand for
protein and increased protein synthesis. The important questions,
how much protein is required to enable dietary protein to be
handled adequately, or how much protein is required to enable
changes in energy intake to be handled, have never been answered
clearly.
The basis of the
assumptions which are drawn presumes that all other nutrient
needs have been adequately satisfied, and this is reasonable for
the purposes of the discussion. However, it is important to
appreciate that the efficiency of utilization of energy overall
is influenced by the availability of micronutrients (KLEIBER,
1945; JACKSON and WOOTTON, 1990), and the pattern of deposition
of protein may be determined by the availability of specific
nutrients.
The general principle can be clearly enunciated, based upon the findings of studies carried out in adults being repleted during total parenteral nutrition (RUDMAN et al., 1975). With energy available for growth, lean tissue is preferentially deposited, provided that the components of lean tissue are available in adequate amounts, and the deposition of adipose tissue represents a limitation of one or another component of lean tissue, thereby acting as a constraint on lean tissue deposition. Furthermore, the pattern of lean tissue formation may be determined by the availability of specific amino acids or micronutrients.
Rats
exposed to a diet low in protein and iron, for example, have
differential patterns of tissue protein repletion depending upon
whether or not protein or iron and protein are provided in the
recovery diet (BEARD), HUEBERS and FINCH, 1984). In rats exposed
to tumour necrosis factor- at the pattern of acute phase proteins
synthesised may be critically determined by the relative
availability of dispensable amino acids in the diet (PATHIRANA
and GRUMBLE, 1992). Therefore, in the face of competitive
functional demands, the utilisation of specific nutrients will be
determined by the relative availability of all other nutrients in
relation to the overall demand.
A diet consisting of
proteins of poor quality may be adequate, if ingested in
sufficient amounts, i.e., to the point where the intake of the
limiting amino acid is satisfied. As the level of food intake is
determined by the demand for energy, the overall intake of
protein is determined by energy expenditure. Therefore, for
populations which take a diet containing proteins of poor
quality, the level of habitual activity will play an important
part in determining the protein adequacy of the diet, and protein
quality will represent a greater problem if the level of activity
is reduced. This problem is likely to be more evident with
ageing, because of the reduced intake. Contrary to popular
belief, this is less likely to be a specific problem in
childhood, even if the demands for growth are increased
significantly, because the demand for energy is sufficiently high
to ensure a relatively generous intake of protein for all but the
most severely limiting diets.
It is often presumed that
ROSE (1938) presented a dichotomous classification of amino acids
as essential or non-essential. This has been taken, incorrectly,
to imply that the dispensable (non-essential) amino acids can
always be made in sufficient quantities to satisfy the metabolic
demand, and that the indispensable (essential) amino acids can
never be made in the body under any circumstances. There is
increasing evidence that the overall metabolic demand has to be
taken into consideration in determining the extent to which
endogenous synthesis of amino acids is sufficient, and most of
the group of nonessential amino acids might become conditionally
essential under some conditions (Table 1; JACKSON, 1992).
It is reasonable to consider the extent to which there may be any
endogenous formation of essential amino acids in the body (see
below). The variable usage and difference of opinion about the
appropriateness of terms such as essential or indispensable,
non-essential nitrogen or non-specific nitrogen, serve to
emphasise the conceptual confusion that exists about the
metabolism of these specific compounds and the absence of a
clear, agreed framework as to their meaning.
Table 1. Classification of amino acids
Essential |
Conditionally essential |
Non-essential |
Leucine |
Glutamate |
|
Isoleucine |
Alanine |
|
Valine |
Aspartate |
|
Glutamine |
||
Arginine |
||
Proline |
||
Histidine |
||
Tryptophan |
||
Phenylalanine |
Tyrosine |
|
Methionine |
Cysteine |
|
Taurine |
||
Threonine |
Glycine |
|
Serine |
||
Lysine |
3.1. Present perception of nitrogen disposal
3.2. Urea production
3.3. Urea excretion
3.4. Salvaged urea nitrogen
3.5. The 'effective dietary intake' of nitrogen
3.6. Limits of adaptation to low-protein diets
3.7. Implications of salvaged urea nitrogen
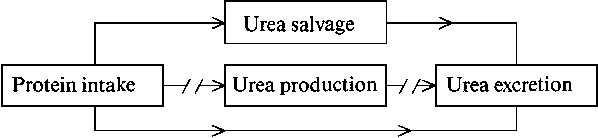
The model presented by Millward makes a number of assumptions, and I would like to consider the following in greater detail:
(a) Obligatory, irreversible amino acid utilisation in non-protein biosynthetic pathways leads to obligatory urea-N and non-urea-N excretion.
(b) Regulatory amino acid oxidation leads to urea production and ammonia excretion.
(c) It is possible to predict that oxidative losses decrease in direct relation to a decrease in protein intake.
Central to the model is the
idea that the oxidation of amino acids is a primary feature of
homeostatic control, both for obligatory nitrogen excretion and
for regulatory amino acid oxidation.
![]()
However, our understanding of the nature and control of these processes is only developed to a limited extent. As it stands, the interpretation of the model is based upon a concept of amino acid oxidation which is outdated and probably incorrect. The model assumes that the rate of oxidation of amino acids is directly related to the level of dietary protein intake. On higher intakes oxidation is increased, whereas on a lower intake the rate of oxidation is decreased. This idea is derived either from in vivo measurements of the rate of nitrogen or urea excretion as dietary protein is increased or decreased, or from in vitro studies in which urea production is related directly to the concentration of substrate presented to liver cells. However, we know from measurements of protein turnover that external balance is only a pale shadow of the internal exchange of amino acids (WATERLOW, 1984b) and nitrogen, and we should therefore be prepared to allow a more complex internal relationship for amino acid oxidation and urea kinetics.
Amino acid
oxidation consists of two processes which must be directly
related, the disposal of the carbon skeleton through oxidation to
CO2 and water, and the disposal of the amino group,
predominantly as urea. There have been very few studies in which
the rates of the two processes have been compared directly. As
pointed out by Millward, when direct comparisons are made between
measurements of carbon flux and of apparent nitrogen flux (as
urea excretion), concordance is not always seen, and this may be
especially true at lower protein intakes. Considering the very
large numbers of studies in which urinary urea excretion has been
used as an index of amino acid oxidation or protein metabolism,
measurements of the relationship between dietary protein and urea
production in vivo are very few (HIBBERT and JACKSON,
1991), and poorly characterised. There are studies in which the
rate of urea excretion has been used as a proxy for urea
production (for example RAFOTH and ONSTADT, 1975), and this study
has been used as justification for the presumption that urea
production is determined directly by protein intake (WALSER,
1981). Therefore, it is worth looking critically at this point.
We have a series of 51 measurements of urea kinetics in normal
adults on adequate protein intakes which vary over a threefold
range in excess of the minimum physiological protein requirement,
35 g/d. From these data it is possible to make a series of
propositions.
The prevailing concept is
that urea production is directly proportional to the dietary
intake of nitrogen. It is known that urea production is
controlled by two main factors: the activity of carbamoyl
phosphate synthetase I through the availability of N-acetyl
glutamate, and the level and activity of the urea cycle enzymes,
with arginosuccinate synthetase probably being rate limiting
(MEIJER, LAMERS and CHAMULEAU, 1990). DAS and WATERLOW (1974)
have shown that, in response to a change in dietary protein from
4 to 14% of intake, the activity of these enzymes increases to a
new steady state within 30 hours, and vice versa.
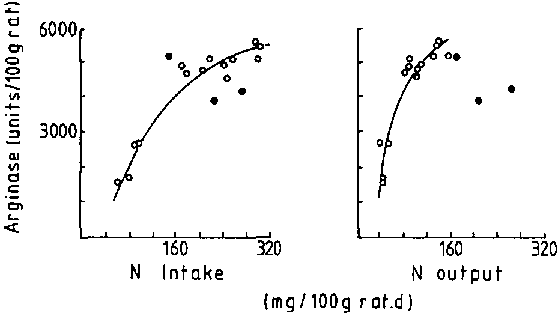
Figure 1 shows that the pattern of response is curvilinear, approaching an asymptote at the higher level of intake. Within a sufficiently large range of intakes, the activity of the enzyme approximates linearity, but for a considerable range of intakes there is little change in overall activity (160 to 320 mg/100 g rat/d).
![]()
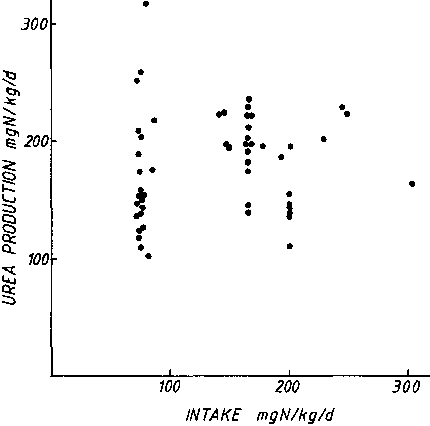
Figure 2 shows that in normal adult humans there is no demonstrable association between urea production and dietary intake over an adequate range of intakes, in excess of 35 g protein/d. Therefore, over habitual levels of intake, there is little variation with protein intake in the activity of the urea cycle enzymes or the production rate of urea. DAS and WATERLOW (1974) showed that arginase activity was not influenced by dietary protein quality, represented as a change in dietary protein from casein to gelatin Therefore it can be concluded that:
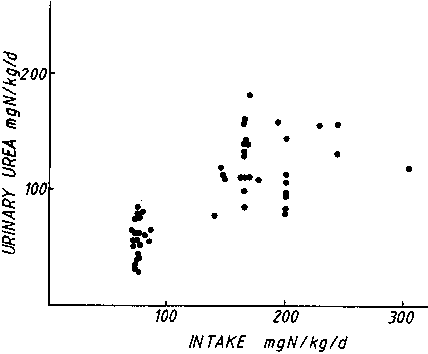
Urea excretion is thought
to be directly proportional to dietary intake of protein and to
urea production. Figure 1 shows that urinary nitrogen
excretion does change with the activity of the urea cycle enzyme
arginase, but the relationship is not linear, and over a normal
range of intakes and losses (above 80 mg/100 g rat/d) the
relationship is not impressive. Figure 3 shows that in
adult humans there is a relationship between intake and excretion
at an intake above 35 g protein/d.
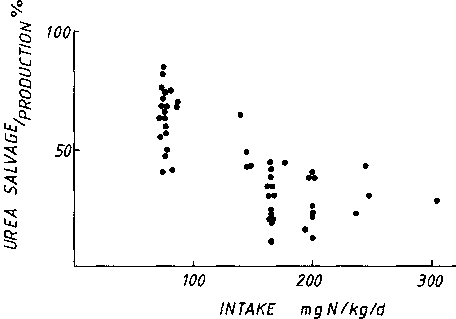
Urea salvage was determined as the difference between urea production and urinary urea excretion in normal adults taking diets providing in excess of 35 g protein/d.
Table 2. Using the same method, urea kinetics were measured on 51 occasions in normal adults ingesting protein within the adequate range of intakes from 35 g/d. The relationships between different aspects of urea kinetics were determined by linear regression analysis
r |
P |
|
Intake compared with: |
||
Production |
0.076 |
0.547 |
Excretion |
0.750 |
<0.0001 |
Salvage |
-0.557 |
0.0001 |
Salvage/Production |
-0.763 |
<0.0001 |
Production compared with: |
||
Excretion |
0.351 |
0.012 |
Salvage |
0.624 |
<0.0001 |
Intake plus salvage |
0.663 |
<0.0001 |
As shown in Table 2, the relationship between nitrogen intake and urea excretion is much stronger than that between urea production and urea excretion. From this we can conclude that, although there is a relationship between nitrogen intake and urea excretion, this does not work solely, or necessarily directly, through urea production, and there are other important determinants than intake which influence production. Therefore:

The urea produced, which is
not excreted, is salvaged through the metabolic activity of the
colonic microflora, although the factors which control this
process are poorly defined (DANIELSEN and JACKSON, 1992). Figure
4 shows that the rate of salvage is responsive to the dietary
protein intake. As the protein decreases, there is an increase in
the proportion of urea-N produced which is salvaged, with 60 to
70% of production being salvaged on an intake of 35 g protein,
around the physiological minimum requirement. The converse is
that, as the intake falls, the proportion of urea production
which is excreted falls. Hence, in part, the extent to which the
urea produced is excreted, is determined by the extent of
salvage. Therefore:
The salvaged urea-N
contributes as an internally generated source of nitrogen to the
'effective dietary intake', which is represented as 'intake plus
salvage'. Figure 5 shows the relationship between urea
production and the sum of intake and salvage. Whereas there was
no relationship between production and intake, there is a very
close relationship between production and 'effective dietary
intake'. A large number of in vitro studies have shown
that the rate of urea production is determined by the substrate
available (MEIJER et al., 1990). From the present data we
can conclude that the 'effective dietary intake' is more likely
to determine the rate of urea production in vivo than the
intake of itself. There is still controversy as to the form in
which the salvaged urea-N is made available to the host, but
increasingly it seems likely that essential and non-essential
amino acids, formed by the microflora, are available to the host
in functionally significant amounts (JACKSON, 1983).
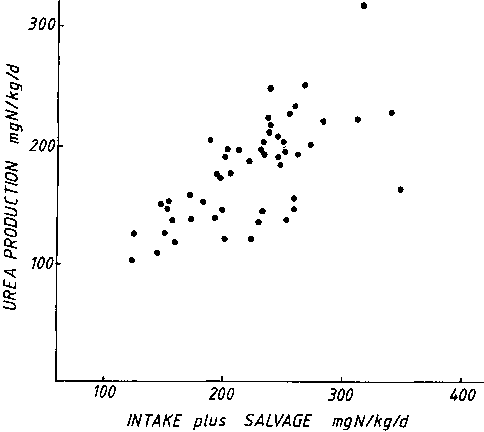
The effective nitrogen intake was related to the rate of urea production in normal adults taking diets which provided in excess of 35 g protein/d.
Figure 1 shows that,
when dietary nitrogen becomes inadequate in the rat, there is a
marked fall in the activity of arginase, with a less dramatic
decrease in nitrogen output. Hence, the internal nitrogen
dynamics are affected to a greater extent than is obvious from
the external balance.
Figure 6 is based upon the data from two separate studies and shows that the same principle applies to the human (LANGRAN et al., 1992; DANIELSEN and JACKSON, 1992). When the protein intake was reduced to 30 g/d, urea excretion increased and nitrogen balance could no longer be maintained. Compared with 35 g protein, there was a significant fall in urea production and significant decrease in the rate at which urea-N was salvaged.
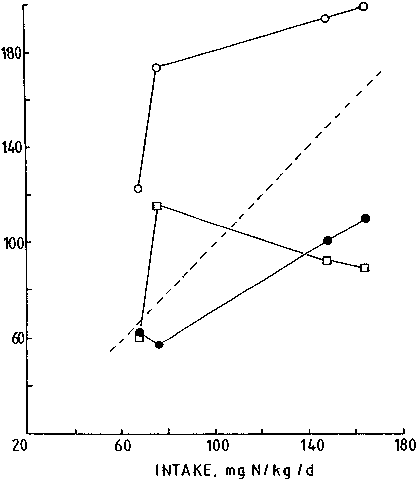
The primary determinant of these changes is unclear, but we have suggested that the ability to maintain urea production as the primary drive to the salvage mechanism may be the most important consideration (DANIELSEN and JACKSON, 1992). This conclusion is based upon, and serves to explain, the benefit in nitrogen balance observed when so-called 'non-essential nitrogen' or 'non-specific nitrogen' is added to the diet. If non-essential nitrogen were used to drive urea production, then the salvage system could be maintained as follows:
This
possibility remains a speculation in the absence of any formal
study.
MILLWARD and RIVERS (1988)
discuss in some detail the relative sparing effect of
non-specific nitrogen on the requirements for indispensable amino
acids. They review the literature which shows that the addition
of non-essential nitrogen to the diet efficiently saves
indispensable amino acids. So, for example, nitrogen balance can
be maintained in a 70 kg man on a diet containing 5 to 6 g
nitrogen, when indispensable amino acids provide 2.5 to 3 g
nitrogen. If the indispensable amino acid content is reduced to
around 1 g nitrogen, balance may be restored by increasing the
total nitrogen content to 8 to 10 g by the addition of
non-essential nitrogen. The data suggest that within these
figures lies an area of metabolic exchange of considerable
importance.
Based upon the data shown in Figure 6, the 'effective nitrogen intake' (intake plus salvage) is 2.5 times the intake. YOUNG and PELLETT (1990) have argued that the apparent requirements for indispensable amino acids (based upon determinations of internal balance using rates of oxidation of the carbon skeleton) are 2 to 3 times that determined from studies in which external nitrogen balance has been used as the criterion of adequacy. The extent to which external balance is achieved through the internal salvaging of urea nitrogen as an effective source of indispensable amino acids, remains to be determined.
WALSER (1981) has been at pains to differentiate between the rate of urea appearance (as the rate at which urea is formed from amino acids) and the rate of urea production (as the rate at which urea is produced from amino acids and salvaged urea-N). Reanalysis of the data shown in Figures 2 to 5, using urea appearance rather than urea production, does not change the conclusions in any way. In practice, the return of salvaged urea-N to urea synthesis represents only a very small proportion of the overall production, less so on lower than on higher protein intakes.
Urea production is clearly associated with the process of amino acid oxidation. The data on urea kinetics suggest that maintaining a certain level of urea production and salvaging of urea-N might be important for maintaining nitrogen balance. If this were to be so, then this represents direct evidence that the oxidative process, rather than oxidative losses, is an integral part of homeostatic control. It further raises the question as to the extent to which the oxidative process in itself, acting as a source of nitrogen for urea synthesis, is central to overall regulatory control. It would be interesting to know whether Millward considers that the 'anabolic drive' might be related in any way to the metabolic benefit of maintaining urea production at a relatively high value. We have no measurements of urea kinetics in individuals on a diet providing adequate energy and zero protein. However, with a total fast the salvage system remains functional. The salvage system is not dependent upon a dietary intake, as patients on total parenteral nutrition have enhanced rates of salvage of urea-N (MORAN, KARRAN and JACKSON, 1991).
In situations of growth, there is net positive nitrogen balance, and the metabolic demand for nitrogen is increased in association with increases in protein synthesis and protein degradation (WATERLOW and JACKSON, 1981). We have found that during normal growth in infancy, compared with catch-up growth from malnutrition, the relationships of urea kinetics are changed. There is a substantial enhancement of the salvage of urea-N on diets which provide relatively generous amounts of protein (JACKSON et al., 1990; WHEELER, JACKSON and GRIFFITHS, 1991). In all the situations we have studied, in which there is a relative increase in demand over the dietary provision of nitrogen, there is enhanced salvaging of urea-N. Taken together, the data argue in favour of the salvage process playing an integral part in overall nitrogen homeostasis.
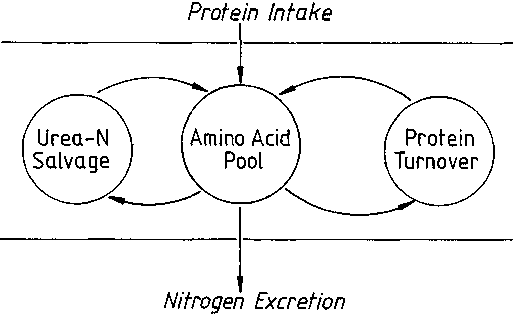
Within the body, dynamic relations in nitrogen metabolism take place through the amino acid pool, which receives amino acids from the protein in the diet and is the precursor for nitrogen excretion, predominantly as urea. There are two major cycles within the body: the protein cycle in which amino acids move into and out of protein through the processes of protein synthesis and degradation, protein turnover; and the nitrogen cycle in which urea-N moves into the bowel and is salvaged as metabolically useful nitrogen. A complete understanding of protein and nitrogen metabolism requires accurate characterization of each of these major processes.