1. Introduction
2. The problem in defining requirements
3. Protein quality
4. The maintenance requirement (MR)
5.Diurnal cycling: the Millward-Rivers model
6. Theoretical basis of the MIT tracer balance studies
7. Technical problems of tracer balance studies
8. Results of the MIT tracer balance studies
9. Relation between leucine oxidation and nitrogen excretion
10. Factors relating to the design of tracer balance experiments
11. Breakpoint analysis
12. Effect of protein/amino acid intake on protein synthesis and breakdown
13. The colon: losses or gains?
14. Conclusion
References
Discussion
References
JC Waterlow
Centre for Human Nutrition, London School of Hygiene and Tropical Medicine, 2 Taviton Street, London, WC1H OBT, UK
Descriptors: indispensable
amino acids, requirements, carbon balance, leucine
Until less than a decade ago all quantitative
estimates of human requirements for protein and indispensable amino acids (IAAs) were
derived from measurements of nitrogen balance. The FAO/WHO/UNU Expert Consultation, which
met in Rome in 1981 (henceforth referred to as the Rome Committee) and which reported in
1985, reviewed the extensive short-term and long term balance studies on healthy young men
and women that had been carried out by Young and Scrimshaw at MIT, by Calloway at Berkeley
and many others. The results of selected studies, summarized in the Rome report, were
fairly consistent in establishing the average total protein requirement, in terms of egg
or beef protein, as 0.625 g protein/kg/d with a mean coefficient of variation (CV) of
12.5%. Studies with typical mixed diets from a variety of countries gave a mean
requirement of 0.745 g/kg/d, with a mean CV within studies of 16.3%. Long-term studies,
carried on for 2-3 months, showed that balances were marginal or negative in most
individuals on intakes slightly below 0.6 g/kg/d. The matter of the total protein
requirement thus seemed to have reached a reasonable solution, but a question mark still
hung over the IAA requirements and the assessment of protein quality.
For the IAAs the Rome Committee based their recommendations on the balance studies of Rose (1957). These estimates (Table 1A) were so low that in the view of the Rome Committee no adult diet anywhere in the world would be limited by its IAA content. In other words, protein quality was not a problem for adults. Leverton's work on young women led to similar values (Leverton, 1959).
Young et al (1989) have stressed the drawbacks of the nitrogen balance method on which these results were based (Table 2). To those listed in the Table should be added the unrealistically high positive balances almost universally obtained with high intakes of nitrogen. Young and coworkers therefore developed a radically new approach, based on the availability of amino acids labelled with stable isotopes. They argued that whereas the classical balance experiment involves determining the amount of amino acid needed to replace losses as measured by N excretion, it would be equally valid to determine the losses by measuring the oxidation of carbon from a labelled amino acid. The general procedure was to infuse the test amino acid labelled with 13C in the 1-carbon position and determine the amount of amino acid oxidized from the output of 13CO2 divided by the isotope abundance in plasma. The details of the method, which has been called the 'tracer balance', and its implications have been discussed by Young (1987), Young et al (1989), Young and Marchini (1990) and Young (1991). The approach was initially applied to determine the requirements for leucine (Meguid et al, 1986a); lysine (Meredith et al, 1986); valine (Meguid et al, 1986b); and threonine (Zhao et al, 1986). The requirement of adults for these amino acids determined by tracer balance turned out to be two to three times greater than the estimates in the Rome report (Table 1). Young has argued in support of these new figures that their pattern (ma amino acid per g protein) is very similar to that of tissue protein and also to the requirement pattern of the pre-school child as proposed by the Rome Committee (Table 1B). These points are considered in more detail in sections 3 and 4.
Correspondence: JC Waterlow, 15 Hillgate Street, London W8 7SP, UK.
In 1989 an Expert Consultation of FAO/WHO on the evaluation of protein quality, impressed by the evidence against the low estimates of adult IAA requirements proposed by the Rome Committee, recommended as a provisional measure that the pattern of IAA requirements of the pre-school child, as set out by FAO/WHO/UNU (1985), should be adopted for all ages other than infants (FAO/WHO, 1990). This pattern is similar to that suggested by the MIT workers.*
* In the text and tables that follow, all references to the FAO pattern or to FAO levels of IAA requirements relate to the pattern of requirement levels proposed by FAO/WHO/UNU (1985).
Millward has pointed out an inconsistency in the Rome report, in that much larger absolute amounts of IAAs were recommended for pre-school children than for adults, even though by 2 years of age the growth component, which requires net protein deposition, is already small compared with the maintenance component (e.g. Millward & Rivers, 1988). The new proposals would meet this point by increasing the adult IAA requirements rather than by reducing those of children.
An analysis of diets in developing countries led to the conclusion that with the new estimates lysine was likely to be limiting in cereal-based diets, and that for an individual to have an intake that met the requirement it would be necessary for about 40% of his protein intake to be derived from animal sources or legumes (Young & Pellett, 1990). Since in many developing countries cereals are the main source of protein, it was concluded that large numbers of people in these countries would be at risk of having deficient intakes of lysine. Young & Pellett admitted that '... individuals consuming diets that we have characterized as being of poor quality have survived under these conditions. We do not know, however, whether this is because our predictions are invalid, whether a relatively benign physiological adaptation has occurred, or whether there has been a functionally costly accommodation to lower than apparently desirable amino acid (or lysine) intake levels'. It is worth noting, however, that the calculations were made for populations of reference body weight, with no allowance for the much lower body weights prevailing in developing countries.
Table 1A Indispensable amino acid requirements: per unit body weight (mg/kg/d)
FAO/WHO/UNU
adultsa |
MIT new
estimatesb |
Predicted
from obligatory lossesb |
FAO/WHO/UNU
pre-school chida |
|
Leucine |
14 |
40 |
39 |
73 |
Lysine |
12 |
30 |
42 |
64 |
Threonine |
7 |
15 |
21 |
37 |
Valine |
10 |
20 |
24 |
38 |
Methionine |
13 |
16 |
||
Methionine +
cystine |
13 |
27 |
||
Safe level of
protein (g/kg/d) |
0.75 |
1.1 |
a From FAO/WHO/UNU
(1985).
b Young et al (1989). Average
requirements.
Table 1B Indispensable amino acid requirements: scoring pattern (mg/g protein)
FAO/WHO/UNU
adultsa |
MIT new
estimatesa |
FAO/WHO/UNU
pre-school childb |
|
Leucine |
19 |
53 |
66 |
Lysine |
16 |
40 |
58 |
Threonine |
9 |
20 |
34 |
Valine |
13 |
26 |
35 |
Methionine +
cystine |
17 |
17 |
25 |
Assuming safe level of total
protein:
a 0.75 g/kg/d.
b 1.1 g/kg/d.
These new recommendations of the MIT group have been strongly criticized by Millward and coworkers (Millward & Rivers, 1988; Millward et al, 1989; Millward, 1992, 1994), but mainly on conceptual and technical grounds, rather than on the basis of different quantitative data. In a series of studies published since 1986, Young et al (1989) have attempted to counter most of these criticisms. It is clear that the new recommendations, if accepted, have important implications for food and agricultural policy. I have therefore tried in this report to examine in detail the experimental observations of the MIT group as well as the ideas of Millward. It has also seemed necessary to consider more general questions, such as what is implied by the term 'requirement', the problem of adaptation, and the still mysterious mechanism by which intake and output are brought into balance.
Table 2 Sources of error in nitrogen balance studies used to estimate adults' amino acid requirements
Issue |
Comment |
Nitrogen balance |
Can be obtained at
various levels of intake and does not necessarily indicate adequacy of intake |
Technical errors |
Tendency to
overestimate N balance and therefore underestimate requirements |
Criterion of
balance |
Need to allow for
miscellaneous unmeasured losses |
High energy intakes |
Excess energy will
improve N balance and thus underestimate requirements at maintenance energy intakes |
Sensitivity and
precision |
Changes in body N
balance may not be detected promptly or with precision |
Validation |
No satisfactory
validation available |
After Young et al (1989).
At the meeting of the FAO/WHO Expert Committee on
Protein Requirements in 1963 (FAO/WHO, 1965) I maintained that there was only one level of
requirement worth talking about - the minimal requirement. I am now persuaded that that
view is too restricted because it was confined to results obtained by nitrogen balance.
Further, it was suggested that the requirements of all men are more or less equal, to
which a committee member from former Czechoslovakia replied 'I am not so sure'. Nicol
& Phillips wrote in 1976: 'The protein requirements of all apparently healthy men can
only be established in the context of their ecological, socioeconomic and nutritional
backgrounds'. Thus as long as 30 years ago doubts were being expressed about the way in
which protein requirements should be formulated.
Millward said 'The established perception of the nature of protein requirements is inadequate' (Millward et al, 1990). For him there are three levels of requirement: the optimal, the operational and the minimal. The optimal requirement would be determined by functional criteria such as good health, growth, resistance to disease. These criteria are hard to define, although studies of immune status could be used at the population level. Chittenden lived a healthy and active life for many years on a protein intake of about 30 g per day. He maintained that the high protein intakes recommended by Voit (about 120 g per day) constituted 'individual and racial suicide'. This is the only example I know of in which health has been the criterion for recommending a specific level of protein intake.* It is an important task for the future to search for correlations between protein intake and functional criteria that can be stated in quantitative terms. For example, there is increasing evidence that linear growth in children may be influenced by diets that provide good quality protein (Allen, 1994; Golden, 1994), although we do not know whether the effect is due to vitamins, minerals or amino acids. Golden (1994) has put forward a convincing hypothesis for the role of sulphur amino acids.
* Chittenden observed that in soldiers and athletes who had been living on a generally high protein diet, change to a mainly vegetarian diet providing 0.75 g protein/kg/d for 5 months led to an increase of 38% in strength and work performance by 15 tests (Millward, 1994).
The operational requirement, a term introduced by Millward, although it has overtones of the NPUop of Miller & Payne (1961), will be discussed below in relation to the Millward-Rivers model. It takes account of the fact that nitrogen balance can be achieved over a wide range of protein intakes. This has long been recognized, at least since the time of Folin (1905), but I think it is fair to say that we still do not know how this balance is achieved (Waterlow, 1994).
The minimal requirement, which has been the object of innumerable measurements, is, as its name implies, the lowest level of protein or amino acid intake at which N balance can be achieved and maintained. The work of Sukhatme & Margen (1978), which at one time had a good deal of influence, seemed to imply that this minimal level could be variable in an individual. Millward et al (1989) have said that their work implies 'a regulatory mechanism which adjusts daily N balance over a period of several days ... adaptive mechanisms exist which adjust output to balance intake and limit the extent of any loss or gain of body protein. This is an alternative model defining the requirement as a range of intakes over which equilibrium can occur. In contrast, the conventional model is based on an intrinsic requirement which is a fixed function of body weight'.
It is necessary to comment on this statement. The range of intakes over which balance can be achieved is well recognized and the description of an 'alternative model' is unjustified. Sukhatme & Margen's theory of regulation is based on the finding of auto-correlation in urinary N output. This means that if the output on day 2 is lower than on day 1, it will be lower on day 3 than on day 2, and so on. This process would, if continued, lead to zero output (negative correlation) or infinite output (positive correlation). Obviously this does not Occur; after a few days the cycle is reversed. This reversal appears to be caused by random variation (Sukhatme, personal communication). Healy (1989) has criticized the concept of autocorrelation on theoretical grounds; Rand et al (1979) looked for it in a large series of long-term balance studies and found evidence of it in only a small minority. In any case, autocorrelation, if it relies on random variation to maintain a long-term steady state, would seem to be the reverse of a regulatory mechanism. A regulatory mechanism is one which, like a thermostat, manages to maintain a steady state by opposing or reducing the effect of random variations or imposed fluctuations (Waterlow, 1985).
In the statement that 'the conventional model is based on an intrinsic requirement which is a fixed function of body weight', the key words are 'intrinsic' and 'fixed'. All balance studies are conducted on a particular individual at a particular point in time with a particular body weight and total body nitrogen. It seems reasonable to suppose on physiological grounds that the nitrogen losses, which have to be balanced by the requirement, should depend on the body weight, or better, the lean body mass or total body N. However, I know of no studies that have attempted to establish how strong the correlation is, in the way that we have studies relating the BMR to body weight or lean body mass. I find it difficult to believe that such a correlation does not exist, but that in no way rules out the influence of other factors, such as body composition, age, sex and possibly height in relation to weight. For example, Egun & Atinmo (1993) showed that on a Nigerian diet women had a lower protein requirement per kg than men, but it was the same when related to lean body mass. If the measurement of nitrogen balance was as easy as that of BMR, we would be far further.
We have no information about whether the minimum requirement per unit body nitrogen is in fact fixed. Studies in third world countries, where people might be supposed to be existing on low protein intakes, have so far shown no significant differences in obligatory N losses from those found in industrialized countries (Torun et al, 1981; FAO/WHO/UNU, 1985). However, even if there is a strict physiological relationship between the daily obligatory losses and the amount of body N. there is still a possibility for adaptation in the efficiency with which amino acids are used (Nicol & Phillips, 1976). Millward (1992) contends that in the adult there is a set-point for the upper limit of body protein, which is determined by height and frame size. This idea of a set-point seems very reasonable. For example, in the normal adult neither plasma albumin nor haemoglobin concentration can be raised above a certain level by an increase in dietary protein. In experiments with rats, Henry et al (1953) showed that with increasing protein intake total liver protein rose towards an asymptote, with ever diminishing returns on the increased intake.
It is also well recognized that on moving from a higher to a lower protein intake there is a small loss of body protein, about 1% in the human adult (Young et al, 1968). This small loss can apparently be tolerated without harmful effects (Waterlow, 1985). It has been regarded as drawing on 'labile protein stores', but the concept of a store is inappropriate. It is probably better to regard it as a kinetic adjustment that allows constancy of body protein to be maintained at a new setting, the processes of protein synthesis and breakdown needing a little time to adjust to the new level of intake (Waterlow et al, 1978).
There is a further stage of
adaptation. If the intake is too low there will be an exponential loss of body protein
until balance is achieved at a new level of body weight (Waterlow, 1985). For example, if
the requirement for maintenance is taken as 0.1 g N per kg per day, and a 70 kg man is on
a diet that provides 5 g N, or 0.07 g per kg, other things being equal he will lose body N
until his weight has fallen to 50 kg, when he will again be in balance. Of course, other
things may not be equal; nitrogen may be used more efficiently, as suggested by Allison's
work on dogs (Allison, 1951). One may ask, what degree of loss of body N is acceptable? If
the subject initially had a height of 1.75 m and at 70 kg a body mass index (BMI) of 22.8,
at the end of this second stage the BMI would be 16.3, which, according to current
thinking, would be inacceptable (James et al, 1988). Moreover, it appears that such
a loss would not be uniform, but would involve a disproportionate amount of muscle mass,
visceral mass being well maintained (Soares et al, 1991). This is a further reason
why the nitrogen balance at a given point in time cannot be regarded as giving a complete
answer to the question of the protein requirement. If we regard the requirement per unit
body weight as fixed, what is the ideal body weight at which it should be fixed, or is
anything short of Millward's setpoint suboptimal?
In absolute terms the requirement for protein in a
non growing adult is the minimum amount needed to meet that body's metabolic demands for
nitrogen, i.e. to secure N balance, in a particular situation. One aspect of the
particular situation is the energy intake. It has long been recognized that, within
limits, increasing or decreasing energy intake alters N balance by 1.5-2 mg/ kg. It was a
criticism of Rose's original estimates of IAA requirements that the energy intake was
unrealistically high. Therefore all comparative studies of protein quality in recent years
have been made with subjects as far as possible in energy balance.** Obviously also
protein quality can only be measured at low intakes. As Jackson (1995) has pointed out, a
distinction has to be made between nitrogen requirements and protein requirements. A
striking example of the need for this distinction is the breast-fed infant, whose food
contains 30% of its nitrogen as non-protein N (see report on infant protein requirements).
The NPN is usually included with protein in estimates of 'protein' requirements, which
should more accurately be called nitrogen requirements, as they are measured by nitrogen
balance.
** At MIT, for example, the nutritionist estimated the habitual energy intake from a dietary history and added 10%. Subjects were required to maintain their usual level of physical activity throughout the study and to monitor it by an activity diary. Weight changes were monitored and, if any trend was apparent, the energy intake was adjusted.
Jackson's analysis of the older literature shows that the requirement for IAAs is influenced by both the amount and the nature of the non-essential (NEN) component, effectiveness decreasing progressively as the NEN is provided by non-essential amino acids, glycine + glutamic acid, ammonium salts and urea. He has proposed that this gradation reflects the relative capacity of these components to provide substrates for the synthesis of IAAs by gut bacteria (see section 13). Whether or not this is the explanation remains to be seen, but the fact is clear, as shown, for example by the experiments of Kies & Fox (1978) among others. In conventional estimates of protein quality, NEN is provided by the NEAAs, which are readily exchangeable by transamination and to a lesser extent by deamination (glycine, serine). Thus in practice it is assumed that protein quality depends only on the amount and pattern of the IAAs.
Estimates of protein quality by N balance are in fact measures of the efficiency of utilization. If a protein could produce balance at an intake exactly equal to the obligatory loss, the efficiency of utilization would be 100%. In fact the efficiency, corrected for digestibility, is never better than about 80%, even with proteins such as those of egg or beef, which have an IAA pattern very close to that of tissue proteins. The Rome Report used a value of 70%. The reasons for this 'inefficiency' are not clear, and this is an important gap in our knowledge.
Millward et al (1989) have summarized the results of eight studies designed to measure the quality of different proteins by balance measurements at a series of different intakes (Figure 1). It happens that the first two in time of these studies, by Young et al (1973) on egg and by Inoue et al (1974) on wheat gluten, show fairly clear differences in biological value at low protein intakes. These early experiences encouraged Young and Scrimshaw to record the view that 'regardless of the method of measurement, our findings indicate that differences in the quality of dietary protein are important in the protein nutrition of adult man' (Young et al, 1975). The less clear-cut results of the later studies shown in Figure 1 do not mean that there are no differences in quality between different proteins; rather that they may be unimportant in practice with diets containing a mixture of proteins, such as those listed in Table 39 of the Rome Report. On the other hand, if the difference between cereals and animal protein is of practical importance, as claimed by Young & Pellett (1990) because of the difference in lysine content, why did it not show up in the balance studies? Rand et al (1981) calculated that to demonstrate a significant difference in biological value in adult humans would require a totally unrealistic number of subjects. Therefore, if Young's claim is sustained that the tracer balance is more sensitive than the nitrogen balance, it will be an important advance.
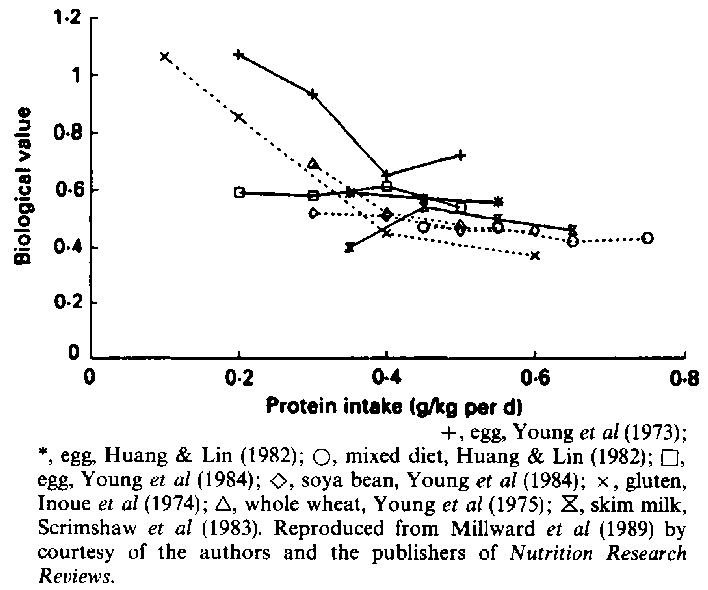
The FAO/WHO reports of 1963 and 1973 gave much attention to the development of an IAA scoring pattern, with its linked concept of a limiting amino acid. The method can be applied to the protein of any diet whose amino acid composition is known, and the measurement is far simpler than the nitrogen balance. It is probably not of great importance whether the scoring pattern is taken to be that of milk, egg, beef, etc. whose relative contents of IAAs are similar to that of human tissue protein. However, the scoring pattern does not tell us anything about the absolute requirement for IAAs as a proportion of the total protein or N requirement. It is agreed that this proportion changes with age, being greater in the growing infant than in older children and adults. There is disagreement about whether the pattern changes with age, and is different for growth and maintenance (section 4). If, as Young maintains, it is not different, and is close to that of tissue protein, then to know the requirement for all IAAs it will be sufficient to know the requirement for one of them.
A third method of examining protein
quality has recently been proposed by Millward et al (1991), as an outcome of his
work on diurnal cycling: the slope of protein deposition vs intake (see section 5).