This is the old United Nations University website. Visit the new site at http://unu.edu
Abstract
Introduction
Methods
Results
Discussion
References
Loretta Brabin, Sarala Nicholas, Alka Gogate, Sharad Gogate, and Alka KarandeLoretta Brabin is head of the Population and Reproductive Health Programme of the Liverpool School of Tropical Medicine. Sarala Nicholas is a statistician who was attached to the Population and Reproductive Health Programme. Alka Gogate is professor and head of the Department of Microbiology, Lokmanya Tilak Municipal Teaching Hospital, which is run by Brihan Mumbai Municipal Corporation in Mumbai, India. Sharad Gogate is a consultant obstetrician at Mahim Maternity Hospital in Mumbai. Alka Karande is executive health officer, Brihan Mumbai Municipal Corporation.Mention of the names of firms and commercial products does not imply endorsement by the United Nations University.
Iron-deficiency anaemia is highly prevalent among women of reproductive age in South-East Asia. In this study, the haemoglobin levels of 2,813 women living in inner-city Mumbai, India, were measured as part of a reproductive health study. Women were recruited over a two-year period at three health facilities providing pregnancy and post-partum services. Five reproductive groups were studied, and the haemoglobin values differed significantly among the groups. Infertile women and women without living children had the highest haemoglobin values (p <.01). However, a least-squares regression analysis of factors affecting haemoglobin status accounted for only 16% of the variability observed. The study concludes that nutritional interventions that focus on reducing fertility or iron supplementation during pregnancy are beneficial, but many women remain iron deficient. Action is needed to improve nutritional status before pregnancy - a policy that is feasible given the current interest in adolescent sexual and reproductive health programmes.
Iron-deficiency anaemia is the most common form of malnutrition in the world and is the eighth leading cause of disease in girls and women in developing countries [1]. Its estimated prevalence in South-East Asia is 50% to 70% [2, 3]. Whereas severe anaemia is closely related to risk of mortality, even mild anaemia carries health risks and reduces the capacity to work [4]. Supplementation of pregnant women remains the cornerstone policy for reducing anaemia among women of reproductive age, because the demands of child-bearing, high fertility rates, and breastfeeding are associated with undernutrition and maternal depletion [5, 6]. Little progress has been made in reducing iron-deficiency anaemia among women in developing countries, in spite of the introduction of iron-supplementation programmes in many of them. In Indonesia, for example, iron supplementation for pregnant women was started some 10 years ago, but the prevalence of anaemia among pregnant women still remains at 63.5% [7].
As part of a study to determine the prevalence of reproductive tract infections and gynaecological morbidity among young women living in inner wards of the city of Mumbai, India, haemoglobin measurements were collected from a large sample of pregnant and non-pregnant women attending health-care centres [8]. In Mumbai a large majority of pregnant women attend centres for antenatal care, and iron and folic acid tablets are provided for daily prophylaxis against nutritional anaemia as an integral part of maternal and child health activities in the Indian Family Welfare Programme [9]. It is recommended that women take 100 tablets of iron and folic acid during pregnancy, and health workers are instructed accordingly. Many of the women were attending for tubal ligations, and we hypothesized that the prevalence of anaemia might be lower among women who were limiting their family size.
Study population
A cross-sectional survey was conducted in Mumbai between October 1993 and December 1995 under the auspices of the Brihan Mumbai Municipal Corporation and with ethical approval from Lokmanya Tilak Teaching Hospital, where all laboratory investigations were undertaken. Since 1988 the Brihan Mumbai Municipal Corporation has extended family health services to all slum areas through the creation of Health Posts and Post-Partum Centres offering health education, preventive services, simple curative care, and family planning. Services are available free at municipal clinics and hospitals. Women aged 35 years or less attending these health facilities were recruited at three inner-city centres that served large slum areas of Mumbai. Women were classified into five groups on the basis of their reasons for inclusion in this study: women being investigated for infertility; women admitted with suspected pelvic inflammatory disease; fertile, non-pregnant women seeking tubal sterilization who had no symptoms of gynaecological disease; pregnant women seeking tubal ligation after early termination of pregnancy; and post-partum women seeking tubal ligation following delivery.
Investigations
Routine haematological tests (erythrocyte sedimentation rate, white blood cell count, and haemoglobin) were performed before laparoscopy or treatment at each clinical centre. Haemoglobin was estimated by the cyanmethaemoglobin method [10]. Anaemia was classified as a haemoglobin concentration less than 12 g/dl for non-pregnant women and less than 11 g/dl for pregnant women [11].
Data analysis
Univariate analysis was undertaken to detect differences in mean haemoglobin using analysis of variance or the Kruskall-Wallis test. The variable “number of years since first pregnancy” was used as an indicator of number of years of exposure to pregnancy. Other variables were age of menarche and socio-economic indicators. Multivariate analysis was performed using ordinary least-squares regression to assess which factors were independently associated with haemoglobin status. All analyses were performed with STATA version 4.1 software [12].
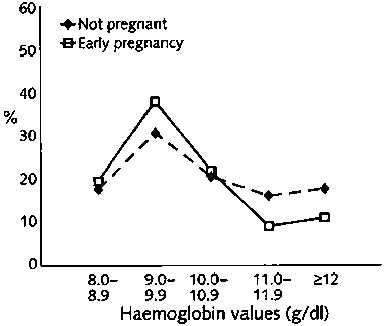
The distribution of haemoglobin values for the five groups of women is shown in figure 1. A total of 2,813 women were studied; 287 were infertile, and 144 were suspected of having pelvic inflammatory disease. Among the remaining women, 588 were not currently pregnant, 908 were in the first trimester of pregnancy, and 886 were requesting sterilization in the post-partum period. There was significant variation among the five groups [p <.001). Whereas most women with a gynaecological problem (infertility or suspected pelvic inflammatory disease) had haemoglobin values of at least 10 g/dl, women who were in early pregnancy or not currently pregnant were more likely to have values less than 10 g/dl (p<.01). About half of post-partum women had values falling between 10.0 and 10.9 g/dl. The mean haemoglobin values (± SD) for the five groups were 11.4 ± 1.2 g/dl for women with infertility, 10.7 ± 1.3 g/dl for those with suspected pelvic inflammatory disease, 10.1 ± 1.5 g/dl for those not currently pregnant, 9.7 ±1.3 g/dl for those in early pregnancy, and 10.4 ± 0.9 g/dl for post-partum women. Severe anaemia (<8.0 g/dl) was not observed.
A univariate analysis of factors likely to confound comparison of mean haemoglobin levels between groups was done. The results are shown in table 1. Of socio-demographic factors, only younger age and having at least 10 years of education were associated with higher mean haemoglobin values. For all the socio-economic categories, differences between the values were small. For the reproductive factors, all showed significant variance between the mean values. The mean age of menarche was 13.0 ± 1.1 years. The mean haemoglobin values were significantly higher for the 14.4% of women who had an early menarche (at 10-12 years of age) and the 11.8% who had a late menarche (at 15 years or more of age). All other significant differences reflected the gap between the high mean haemoglobin values of women who had never been pregnant or had a live birth, and fertile women.
Table 2 shows which reproductive factors remained independently associated with haemoglobin levels after regression analysis. Socio-economic variables are not shown, as only having at least 10 years of education remained significant. The mean haemoglobin concentrations for women in different reproductive categories were significantly different after adjustment for sociodemographic factors. Those who were pregnant or who had a surviving child were significantly more likely to have lower mean haemoglobin levels. The values for women with younger and older ages of menarche also remained significant. However, the regression model accounted for only 16% of the variability in observed haemoglobin status.
This study showed a high prevalence of moderately severe anaemia (<10 g/dl haemoglobin) among young women of reproductive age attending health facilities. Haemoglobin values were measured routinely in each clinic and were not independently validated, so some range of error may be expected. The distribution of values shown in figure 1 is, nonetheless, what would be expected, with the lowest values in pregnant women. The values were highest among women with suspected pelvic inflammatory disease who had impaired fertility, probably as a result of this disease [13]. The proportion of both pregnant and non-pregnant women with moderate anaemia was higher than that recently reported from studies in five other countries [14]. A study in Indonesia reported a 27.9% prevalence of anaemia (<12.0 g/dl haemoglobin) among non-pregnant working women, 21.1% among adolescent girls, and 52.3% (<11.0 g/dl haemoglobin) among pregnant women [15]. In the present study, 82.2% of non-pregnant women and 79.6% of pregnant women fell below recommended values (fig. 1), which represents a staggering level of anaemia.
TABLE 1. Univariate analysis of possible factors confounding haemoglobin status
|
Variable |
Na |
Mean haemoglobin (g/dl) |
SD |
P |
|
|
Sociodemographic |
|||||
|
Age (yr) |
|
|
|
|
|
|
|
<25 |
577 |
10.5 |
1.3 |
<.01 |
|
|
25-29 |
1,282 |
10.2 |
1.3 |
|
|
|
³30 |
954 |
10.1 |
1.3 |
|
|
Years of education |
|
|
|
|
|
|
|
<3 |
1,152 |
10.3 |
1.3 |
<.01 |
|
|
4-9 |
1,184 |
10.2 |
1.4 |
|
|
|
³10 |
475 |
10.4 |
1.4 |
|
|
Religion |
|
|
|
|
|
|
|
Hindu |
2,076 |
10.3 |
1.3 |
.11 |
|
|
Muslim |
342 |
10.3 |
1.3 |
|
|
|
Other |
394 |
10.1 |
1.3 |
|
|
Place of birth |
|
|
|
|
|
|
|
Bombay |
1,139 |
10.2 |
1.3 |
.11 |
|
|
Other |
1,669 |
10.3 |
1.3 |
|
|
No. of household members |
|
|
|
|
|
|
|
<6 |
1,983 |
10.2 |
1.3 |
.83 |
|
|
>6 |
830 |
10.2 |
1.3 |
|
|
Monthly household income (rupees) |
|
|
|
|
|
|
|
< 1,000 |
592 |
10.3 |
1.3 |
.16 |
|
|
1,000-3,000 |
1,648 |
10.2 |
1.3 |
|
|
|
>3,000 |
573 |
10.3 |
1.4 |
|
|
Reproductive |
|||||
|
Age at menarche (yr) |
|
|
|
|
|
|
|
10-12 |
399 |
10.5 |
1.2 |
<.01 |
|
|
13-14 |
2,051 |
10.1 |
1.3 |
|
|
|
>15 |
329 |
10.7 |
1.2 |
|
|
No. of pregnancies |
|
|
|
|
|
|
|
0 |
236 |
11.3 |
1.2 |
<.01 |
|
|
1 |
84 |
11.1 |
1.4 |
|
|
|
2 |
823 |
10.0 |
1.4 |
|
|
|
3 |
1,097 |
10.2 |
1.2 |
|
|
|
4 |
408 |
10.2 |
1.3 |
|
|
|
³5 |
165 |
10.1 |
1.2 |
|
|
Years since first pregnancy |
|
|
|
|
|
|
|
0 |
236 |
11.3 |
1.2 |
<.01 |
|
|
<7 |
1,043 |
10.2 |
1.3 |
|
|
|
³7 |
1,513 |
10.1 |
1.3 |
|
|
Pregnancy outcome |
|
|
|
|
|
|
|
Live births only |
1,959 |
10.1 |
1.3 |
<.01 |
|
|
No live births |
41 |
11.4 |
1.4 |
|
|
|
Varied outcome (live births and/or abortions or
stillbirths) |
577 |
10.3 |
1.3 |
|
a. Numbers vary because data for some women are missing.
FIG. 1. Distribution of haemoglobin values for women in five groups defined by reproductive status (a)
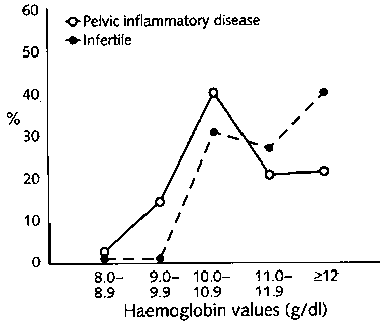
FIG. 1. Distribution of haemoglobin values for women in five groups defined by reproductive status (b)
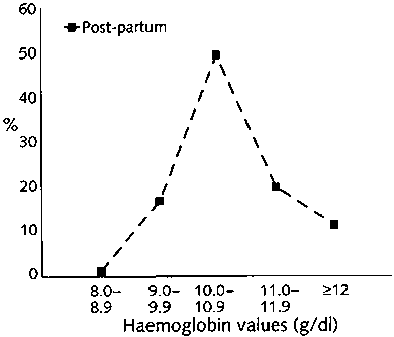
FIG. 1. Distribution of haemoglobin values for women in five groups defined by reproductive status (c)
That women without children had higher haemoglobin values than women with living children (table 2) supports the view that childbirth, lactation, and child-bearing tax a woman’s nutritional condition. However, the magnitude of the effects of child-bearing on haematological status in this population was limited. The regression analysis showed a difference of -0.1 g/dl from the mean baseline haemoglobin value for each live birth, which is small. The explanation for this is probably the low mean number of children born to currently married women in Maharashtra State: 2.95, with a mean of 2.62 children still living [9]. In our study population, women had a mean of three pregnancies over an average reproductive span of 10 years [8]. The short reproductive span is a result of tubal sterilization at an early age (mean, 28 years). Thus, increased child survival, reduced fertility, and short-term measures such as iron supplementation during pregnancy are likely to have mitigated some of the effects of child-bearing on anaemia, and may account for the absence of severe anaemia (<8 g/dl haemoglobin).
The results of this study suggest that in India, interventions that focus on reducing fertility or on iron supplementation during pregnancy will have beneficial nutritional effects but will still leave most women iron deficient. In Mumbai the women studied were largely from poor backgrounds and probably had inadequate diets. However, the problem of undernutrition generally started much earlier in life, with gender discrimination resulting in undernutrition of girls [16-18], which was exacerbated by menstrual iron losses after menarche [19]. The higher haemoglobin values in women who had had either early or late menarche is not surprising, since early menarche is associated with better nutrition and late menarche is associated with fewer years of monthly blood loss. Health promotion to improve the diet of girls and iron supplementation in adolescence are required to redress nutritional deficits and, in the longer term, to reduce anaemia in older women of reproductive age.
Although this policy has been recommended by several authors [4, 15], it has rarely been implemented, probably because adolescents are less easy to reach than pregnant women. This situation is changing, however, because adolescent reproductive health is now an important item on international agendas [20], and governments are starting to draw up national policy guidelines to improve adolescent sexual and reproductive health. Nutritional information and supplementation should be included in these policies, and every effort should be made to link supplementation to other interventions reaching young girls. Although the factors investigated are significant in some cases, it should be emphasized that they leave 84% of the variability unaccounted for.
TABLE 2. Least-squares regression analysis of reproductive factors affecting haemoglobin status
|
Variable |
Difference in mean Hb from baseline value |
SE |
95% CI for mean difference |
P |
|
|
Baseline group |
10.2 |
0.2 |
|
|
|
|
Study groups |
|||||
|
|
Infertility |
1.2 |
0.11 |
0.98 to 1.43 |
<.01 |
|
|
Suspected PID |
0.5 |
0.12 |
0.31 to 0.77 |
<.01 |
|
|
Early pregnancy |
-0.3 |
0.06 |
-0.44 to 0.19 |
<.01 |
|
|
Post-partum |
0.4 |
0.07 |
0.28 to 0.54 |
<.01 |
|
Pregnancy outcomesa |
|||||
|
|
Abortionsb |
0.2 |
0.04 |
0.08 to 0.25 |
<.01 |
|
|
Stillbirths |
0.1 |
0.12 |
-0.15 to 0.33 |
.44 |
|
|
Post-natal deaths |
0.1 |
0.05 |
-0.03 to 0.18 |
.17 |
|
|
Livebirths (children surviving) |
-0.1 |
0.03 |
-0.13 to 0.00 |
.05 |
|
Age at menarche in baseline group (yr) |
|||||
|
³15 |
0.5 |
0.07 |
0.35 to 0.62 |
<.01 |
|
|
10-12 |
0.2 |
0.07 |
0.04 to 0.30 |
.01 |
|
Abbreviations: Hb, haemoglobin; CI, confidence interval; PID, pelvic inflammatory disease.Anaemia surveillance, focusing on moderate to severe anaemia, has been proposed as a way to help donors and governments focus their efforts to reduce anaemia [14], and it can help dispel the misconception that little can be done because anaemia is widespread in all countries. Monitoring haemoglobin levels can draw attention to anaemia as a major public health problem, facilitate health promotion related to improved dietary practices for girls, and provide a relatively simple approach to assessing the nutritional status of women.
a. Mean haemoglobin concentrations were calculated on the basis of women’s histories for any of the listed pregnancy outcomes.
b. Includes women with a history of spontaneous abortion or medical terminations.
Acknowledgements
This research was carried out by the Brihan Mumbai Municipal Corporation and the Population and Reproductive Heath Programme at Liverpool. The research on pelvic inflammatory disease was funded by the Overseas Development Administration of the United Kingdom (now the Department for International Development) with a subsidiary grant from the World Health Organization. Many people contributed to the success of this study and for all their help we are grateful, especially that of Dr. S. Desmukh, Dean of the Lokmanya Tilak Municipal Teaching Hospital, for facilitating work at Sion Hospital. Thanks are also due to Dr. B. Brabin, who provided constructive comments on analysis and interpretation.
1. World Bank. World development report: investing in health. New York: Oxford University Press, 1993.
2. Garcia M, Mason J. Second report of the world nutrition situation. Geneva: United Nations Administrative Committee on Co-ordination/Sub-Committee on Nutrition, 1992.
3. MotherCare. Prevalence of maternal anemia in developing countries. Working Paper No. 7. Arlington, Va, USA: John Snow, 1992.
4. Cohen BJB, Gibor Y. Anaemia and menstrual blood loss. Obstet Gynecol Surv 1980;35:597-618.
5. Winikoff B. The maternal depletion syndrome: clinical diagnosis or eco-demographic condition? Biol Soc 1988;5:163-70.
6. Winkvist A, Rasmussen KM, Habicht J. A new definition of maternal depletion syndrome. Am J Public Health 1992;82:691-4.
7. Muhilal, Sumarno I, Komari. Review of surveys and supplementation studies of anaemia in Indonesia. Food Nutr Bull 1996;17:3-6.
8. Brabin L, Gogate A, Gogate S, Karande A, Khanna R, Dollimore N, de Koning K, Nicholas S, Hart CA. Reproductive tract infections, gynaecological morbidity and HIV seroprevalence among women living in the inner city of Mumbai, India. Bull WHO 1998; 76(3) (in press).
9. International Institute for Population Studies. National family health survey, India 1992-1993. Bombay: International Institute for Population Studies, 1995.
10. Dacie JV, Lewis SM. Practical haematology. 5th ed. Edinburgh: Churchill Livingstone, 1975.
11. Gibson RS. Principles of nutritional assessment. New York: Oxford University Press, 1990.
12. STATA4. Stata Corporation, 702 University Drive East, College Station, TX 77840, USA.
13. Templeton A, ed. The prevention of pelvic infection. London: RCOG Press, 1996.
14. Stoltzfus RJ. Rethinking anaemia surveillance. Lancet 1997;349:1764-6.
15. Gross R, Angeles-Agdeppa I, Schultink WJ, Dillon D, Sastroamidjojo S. Daily versus weekly iron supplementation: programmatic and economic implications for Indonesia. Food Nutr Bull 1997;18:64-70.
16. Ravindran S. Health implications of sex discrimination in childhood. WHO/UNICEF/FHE 86.2. Geneva: WHO and UNICEF, 1986.
17. Das Gupta M. Selective discrimination among female children in rural Punjab, India. Pop Dev Rev 1987;13:77-100.
18. Nielsen BB, Liljestrand J, Hedegaard M, Thilsted H, Joseph A. Reproductive pattern, perinatal mortality and sex preference in rural Tamil Nadu, South India: community based, cross sectional study BMJ 1997;314:1521-4.
19. Hallberg L, Hultén L. Iron requirements, iron balance and iron deficiency in menstruating and pregnant women. In: Hallberg L, Asp NG, eds. Iron nutrition in health and disease. London: John Libbey, 1996:165 - 82.
20. Department for International Development. Eliminating world poverty: a challenge for the 21st century. White Paper. London: UK Government, November 1997.