This is the old United Nations University website. Visit the new site at http://unu.edu
Nutrition and health from womb to tomb
Abstract
Introduction
Examples of nutritional disorders with lasting consequences
Synergism of nutrition and infection
Control and eradication of infectious diseases
Disappearance of some classic deficiency diseases
Diet and chronic disease
Significance of maternal nutrition
Foetal and infant nutrition and adult disease
Lessons from a study of civil war veterans
Lifestyle and the ageing process
Some significant non-nutritional behaviours of health importance
Physical environment and health
Improving on nature
Conclusion
References
Nevin S. Scrimshaw
The author is an Institute Professor Emeritus of the Massachusetts Institute of Technology in Cambridge, Massachusetts, USA, and director of the Food and Nutrition Programme for Human and Social Development of the United Nations University.Reprinted, with expanded tables and figures, with permission from Nutrition Today 1996; 31(2): 55-67.
Fulfilment of the genetic potential of individuals is impaired by malnutrition and other environmental factors throughout life. The impact of nutritional factors in early childhood helps to explain why the known adult risk factors, demonstrable in populations, are poor predictors of cardiovascular disease in individuals. The combined findings of early and later risk factors strengthen the conclusion that the bulk of so-called degenerative diseases and many other functional impairments in adult life occur because of environmental factors, of which diet may be the most important. The apparent increased susceptibility to degenerative diseases of adults born small-for-date suggests that the increase in meat, fat, and calorie consumption with rising affluence is particularly hazardous for formerly poorly nourished populations. Articles confirming the nutritional origins of a wide range of diseases and disabilities at all ages increasingly predominate in the health-related scientific literature. Recognition that so much disease can be prevented or delayed by improved diet and related lifestyles constitutes a new paradigm applicable to the populations of both developing and industrialized countries.
In one of the Oz books, the author, Frank Baum, poses an interesting ethical question. The King of the Realm had given his wife and children to the Nome King in exchange for a long life. The King of the Realm subsequently destroyed his life by leaping into the sea. Was he cheated by the wizard, or had he been given a long life that he wantonly destroyed?
The theme of this essay is that most, but of course not all, of us are given long lives by nature that we shorten by a variety of destructive behaviours and environmental influences. Poor food habits and nutritional deficiencies and excesses, along with smoking, exposure to infections, toxic factors in the environment, and trauma, are the major determinants of how closely the functional capacity, health, and longevity of individuals match their genetic potential. Newly appreciated is the extent to which nutrition and other factors during pregnancy and infancy influence not only the health of the foetus and the infant but also that of the individual throughout life.
Previous Western generations gradually became aware that infectious diseases arise from the biological, not the spirit or magic, domain. This meant that these diseases could be influenced by human behaviour affecting transmission or susceptibility. This was also a time when nutritional deficiency diseases were being recognized. It slowly became apparent that much could be done to prevent both infectious and nutritional diseases, and that they interact synergistically. Therefore, preventive measures affecting either are mutually reinforcing. Recognition of the relationship between nutrition and chronic degenerative diseases has followed. It is now being increasingly realized that health-related behaviours, both positive and negative, are major determinants of the health status of nearly everyone.
Of course, genetic variation in individual resistance is a factor in nearly all diseases, and some individuals are born with genetic traits and inborn errors of metabolism that can be devastating. Nevertheless, for most persons, health and functional capacity are determined not primarily by their genetic potential but by their health-related behaviours and the quality of their environment. These factors determine not only the length of life, but also its physical quality. This new paradigm recognizes that the frequency and severity of nearly all diseases are strongly influenced by individual and societal behaviours. Thus preventive, not curative, medicine should be given first priority. This essay explores some of the basis for asserting this.
There are a number of readily preventable nutritional deficiencies in young children that drastically impair their long-term mental development, physical capacity, and survival.
Protein-energy malnutrition
In the 1950s and 1960s, it became increasingly evident that there was a synergistic interaction between malnutrition and infections. Frequent episodes of infection worsened the nutritional status of children on marginal diets and were the principal precipitating cause of the stunting, marasmus, and kwashiorkor that were common in many developing countries [1]. Growth failure in the young child occurs wherever chronically inadequate diets prevent catch-up growth following an infectious episode [2]. It results in stunted adults who are physically less productive.
Even more important than the physical stunting is the lasting effect of early childhood protein-energy malnutrition on learning and behaviour. Severe cognitive effects of marasmus and marasmic kwashiorkor have been reported from many countries, including Chile [3], India [4], Jamaica [5], Mexico [6], Peru [7], Yugoslavia [8], and Uganda [9]. For example, in Mexico differences in language scores were particularly strong between children recovered from marasmic kwashiorkor and adequately nourished children living in the same social environment, when these were matched for weight and length at birth. Similar differences in intelligence quotients have been reported between children who have experienced severe malnutrition and their adequately nourished siblings studied at the same age [10]. In Chile, the IQs of children four to seven years after marasmus in infancy were so impaired that they did not overlap with those who had not had marasmus [11].
It gradually became evident that even subclinical protein-energy malnutrition affected learning and behaviour. In both Mexico and Guatemala, Cravioto [12,13] found that pre-school children in the lowest quartile of weight-for-age had significantly poorer performances on various tests of intersensory perception than did those in the highest quartile. No such relationship with weight quartile was observed in children of professionals in middle- and upper-income groups in these countries. In this case, as in others that I will cite, it is not variation in height per se that counts, it is nutritionally induced stunting that robs individuals of their full potential. In Bogota, Colombia, children who were administered a food supplement from birth to three years of age averaged 2.6 cm taller and 642 g heavier than controls and had higher test scores than the control group [14]. There are similar reports from Kenya [15].
Two well-designed studies begun in the late 1960s in Guatemala [16] and Mexico [17] convincingly demonstrated that poor rural breastfed children who received supplementary feeding up to two years of age were taller, had fewer infections, and performed better on appropriate cognitive tests. In the Guatemala study from 1969 to 1977, children in two villages received incaparina, a nutritionally balanced protein-calorie beverage, twice daily for two years, and it was also offered to the mothers during pregnancy. In two other villages, fresco, a protein-free, low-calorie beverage, balanced the stimulation received by the children in the incaparina group.
In the Mexican study, half of the children in the village of Tezonteopan received a supplement of milk with added vitamins and minerals, and all of the children in the village received stimulation visits and medical care [17]. Both studies have been followed up nearly 15 years later and have provided striking confirmation of the lasting benefit of preventing malnutrition at an early age.
In Guatemala, two of the original investigators were able to locate over 2,000 of the original subjects and re-examine them as adolescents and young adults [18, 19]. Although there had been no further intervention, those who had received the incaparina during early childhood had maintained their advantages over the group receiving only fresco. They had completed more years of schooling and had performed significantly better per year of schooling on the Central America achievement test that combined literacy, numerical and general knowledge, Raven’s Progressive Matrices, reading, and vocabulary. Overall, the cognitive gains, which were small at two years of age, could now be characterized as medium to large.
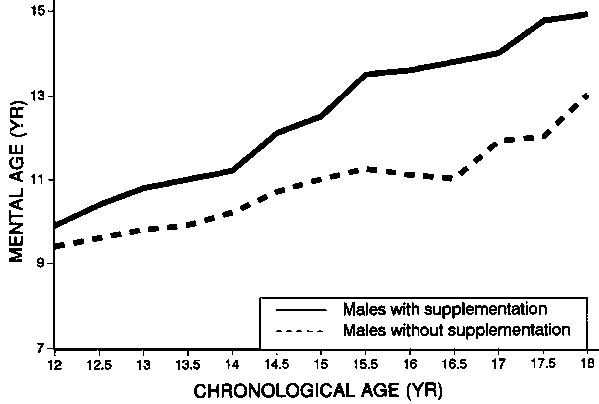
Stimulated by these observations, the Mexican investigators were able to re-examine most of those who participated originally. They found that those who, as young adults, had been supplemented as young children continued to perform better in all respects. Figure 1 shows the higher IQ scores of the supplemented males at all ages up to 18 [20]. The results for females are similar.
A study in Cali, Colombia, showed how difficult it is for an intervention programme to overcome the effects of malnutrition and a slum environment. Preschool slum children were given one to four years of nutritional supplementation and stimulation six days a week [21]. Although each additional year of the combined treatment brought about significant improvement in cognitive test performance compared with control children, even after four years of the programme test scores were 20% lower than in children of middle- and upper-income families not enrolled in the programme. In another study in Cali, supplementary feeding prevented most of the growth loss associated with diarrhea and other infections [22]. A meta-analysis of the six well-controlled field studies, in which infants at nutritional risk received supplementary feeding, showed that supplementary feeding benefited both motor and mental development, depending on age [23].
FIG 1. Effect of early childhood supplemental feeding on later development of male children in Tezonteopan, Mexico. Source: ref. 20
Chronic undernutrition in adults
Chronic undernutrition in adults also has a wide variety of adverse functional consequences [24, 25]. The most convenient indicator of chronic undernutrition is the body mass index (BMI), which is simply height in meters divided by weight in kilograms squared. Almost any functional indicator shows individuals with low BMI to be worse off. For example, table 1 illustrates the association of chronic undernutrition in Indian men with higher mortality [26]. Figure 2 shows the relationship found in Bangladesh between fathers’ BMI and the percentage of time not working due to illness [27]. Similarly, the BMI of Guatemalan wage labourers was shown to be linearly related to the amount of coffee beans picked per day, the amount of sugar cane cut or loaded, and the time taken to weed a given surface area.
Women suffer even more. Figure 3 indicates the relationship between BMI and days of illness among women in Rwanda (P. François, unpublished data, 1990). Low BMI is particularly frequent in Asia, with nearly half of the women in India [28] chronically deficient in dietary energy as judged by low BMI.
TABLE 1. Body mass index (BMI) and mortality in rural Indian men
|
BMI |
Deaths per 1,000 per year |
|
>18.5 |
12.1 |
|
17-18.4 |
13.2 |
|
16-16.9 |
18.9 |
|
<16 |
32.5 |
Source: ref. 26.Nutrient deficiencies
Specific vitamin and mineral deficiencies at all ages have serious and often lasting adverse functional consequences.
Iron deficiency
Iron deficiency is the most widespread nutrition problem in the world today. According to the World Health Organization (WHO), overall rates for iron-deficiency anaemia in developing countries are 26% for men, 42% for women, 46% for school-age children, and 51% for children four years of age or less [29]. Iron-deficiency anaemia has long been associated with weakness and tiredness. It is now also recognized that even mild to moderate iron deficiency without anaemia has adverse consequences. These include decreased physical capacity and work performance of adolescents and adults, reduced growth of children, lowered immune status, increased morbidity from infections in all age groups, and, when severe, also interference with body-temperature regulation [30].
FIG. 2. Relation between BMI and illness among fathers in Bangladesh. Source: ref. 27
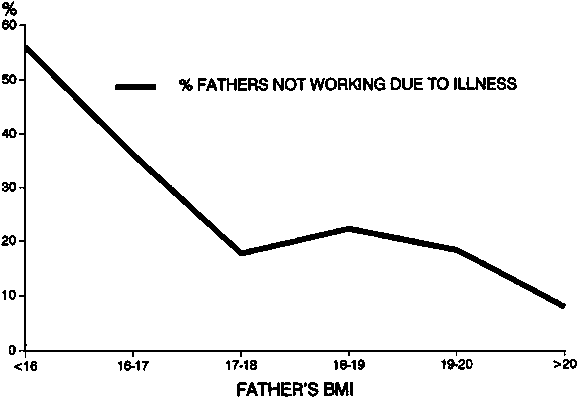
FIG. 3. Relation between BMI and “equivalent days” of illness among women in Rwanda in 1982. CED, Chronic energy deficiency. Source: Unpublished data of P. François, Food and Agriculture Organization, 1990

Iron-deficiency anaemia has also been shown conclusively to delay psychomotor development and impair the cognitive performance of infants in Chile [31], Costa Rica [32, 33], and Guatemala [33]; of pre-school children in Colombia [34], Egypt [35], and Indonesia [35, 36]; of schoolchildren in Thailand [35], Indonesia [37, 38], Egypt [39], and India [40]; and of children [41-44] and adults [42] in the United States. Table 2 shows the relationship between haemoglobin status and Bayley test scores for infants in Chile [31]. A psychomotor development index for infants with and without iron deficiency and anaemia in Costa Rica is shown in figure 4 [32]. The effects of frank anaemia in infancy in Costa Rica were not reversed by iron supplementation and were evident at the time of school entrance, even when anaemia was no longer present [45]. This result was not altered when the analysis was controlled for a comprehensive set of socio-economic factors.
Figure 5 shows the improvement observed in the pioneering study of Pollitt in Cambridge, Massachusetts, USA, on mildly iron-deficient children without anaemia, which aroused initial interest in this topic [44]. Anaemic schoolchildren in Thailand showed poorer performance on Thai language and mathematics tests than did those with low haemoglobin; this was not reversed by iron supplementation [35]. However, in Indonesian schoolchildren whose iron deficiency was relatively mild, iron supplementation resulted in considerable improvement [36]. Figure 6 illustrates the considerable improvement in IQ that was observed [38]. More recent studies have shown that iron-deficient teenage girls have retarded reaction times and other test performances that improve with iron supplementation [46, 47].
TABLE 2. Effect of iron status on Bayley test performance of 12-month-old infants in Chile
|
Index |
Normal a |
Anaemic a |
Sideropenic a |
|
Mental Development (MDI) |
105 ± 9 |
98 ± 9 |
104 ± 9 |
|
Psychomotor Development (PDI) |
101 ± 11 |
90 ± 14 |
100 ± 8 |
Source: ref. 31.FIG. 4. Psychomotor development scores at different levels of iron deficiency among infants in Costa Rica. Source: ref. 32
a. p < .001 for anaemic vs normal and for sideropenic vs anaemic.
Thus, while iron deficiency can impair cognitive performance at all stages of life, the effects of iron-deficiency anaemia in infancy and early childhood are not likely to be reversed by subsequent improvement in iron status. Ten percent of infants in industrialized countries and 30% to 80% of those in developing countries are anaemic at one year of age. These children will have delayed psychomotor development and when they reach school age will have poorer performance on tests of language skills, motor skills, and coordination equivalent to a 5- to 10-point deficit in IQ.
Iron deficiency in the mother during pregnancy increases maternal mortality, prenatal and perinatal infant loss, and prematurity. Moreover, if the mother is iron deficient, her child is born with poor iron reserves and is at greater risk of morbidity and mortality during infancy. Iron deficiency in the child also inhibits growth, impairs immunity, and increases morbidity from infectious disease.
Vitamin A deficiency
About 350,000 infants and young children become blind annually because of vitamin A deficiency, and 70% of these die within one year. WHO estimates that about 40 million children in the world suffer from vitamin A deficiency, although the prevalence of vitamin A deficiency varies greatly among regions and countries [48]. In the last few years, eight studies (summarized in table 3) have examined the effect of vitamin A supplementation of pre-school children on mortality from infectious disease [49]. In six of the studies, there was a decrease in mortality of 30% to 50%. Even though two of the studies found no significant effect, perhaps because other nutrients were limiting, the meta-analysis of the eight studies suggested an average mortality reduction of 33% [49].
As I have emphasized, malnutrition and infection are both major separate contributors to morbidity and mortality. However, in underprivileged populations it is the synergistic interaction between malnutrition and infection that is so dangerous. Malnourished individuals have reduced resistance to infections because both humoral and cell-mediated immunity can be affected. The result is that they have more frequent and severe infections, particularly diarrhoeal and respiratory diseases. Moreover, infections, even when they are mild or subclinical, worsen nutrition by a variety of mechanisms. These include reduced appetite, metabolic nutrient losses in urine, internal diversion of nutrients, and, frequently, reduced absorption of nutrients.
Nutrition is not a factor in all infections. Some affect well-nourished and poorly nourished alike and may have permanent health consequences. Examples of such diseases are poliomyelitis, which can cause lasting muscle weakness and paralysis in children, and hepatitis B. which can lead to liver cancer. Human immunodeficiency virus (HIV) infection nearly always ends disastrously.
FIG. 5. Performance of control and experimental groups of children in Cambridge, Massachusetts, USA, on oddity learning repeated presentation task 1, before (T1) and after (T2) administration of oral iron. Values are means ± SE. Source: ref. 44
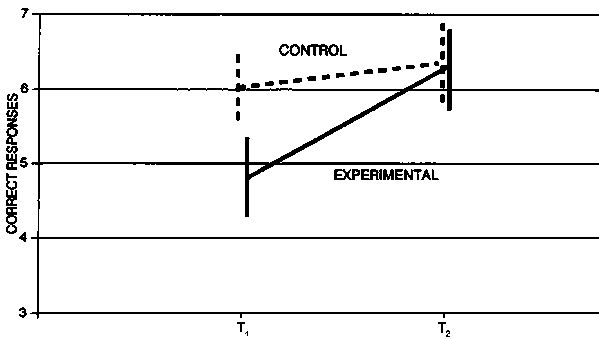
FIG. 6. Effect of iron supplementation on IQ test scores of Indonesian schoolchildren. Source: ref. 38
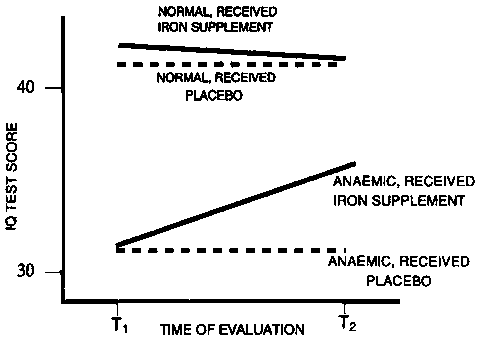
TABLE 3. Effects of eight vitamin A intervention trials on mortality of young children
|
Location of trial |
% relative risk a |
|
Aceh, Indonesia |
73 |
|
Hyderabad, India |
94 |
|
Sarlahi, Nepal |
71 |
|
Bogor, Indonesia |
70 |
|
Sudan |
104 |
|
Jumla, Nepal |
74 |
|
Vast, Ghana |
80 |
|
Tamil Nadu, India |
50 |
Source: ref. 49.
a. Mean relative risk, 77%; 95% confidence limits, 68% to 84%.
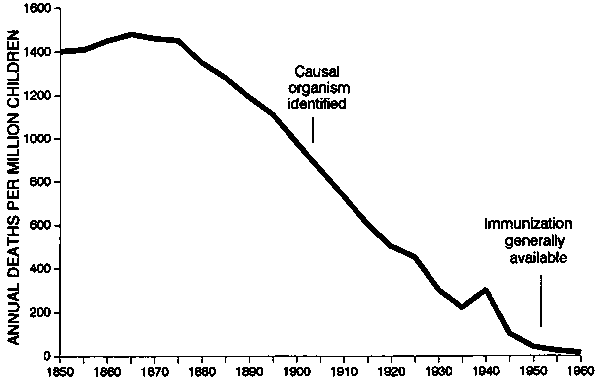
Space does not permit a review of the history and reasons for the decline of infectious disease mortality since the last century. In the late nineteenth and early twentieth centuries, deaths from the common communicable diseases in Europe and North America declined dramatically, well before specific medical therapies were available. Figures 7 and 8, adapted from McKeown [50], show the drop in deaths from tuberculosis and whooping cough in England and Wales over the course of the past century. The fall in death rates from diphtheria, measles, and pneumonia was similar. After analysing the possible factors, McKeown concluded the major reason for most of the decline was improved nutrition.
FIG. 7. Fall in deaths from tuberculosis in England and Wales from 1838 to 1970. Most of the drop occurred before specific treatment or immunization was available. BCG, Bacillus Calmette-Guérin. Source: ref. 50
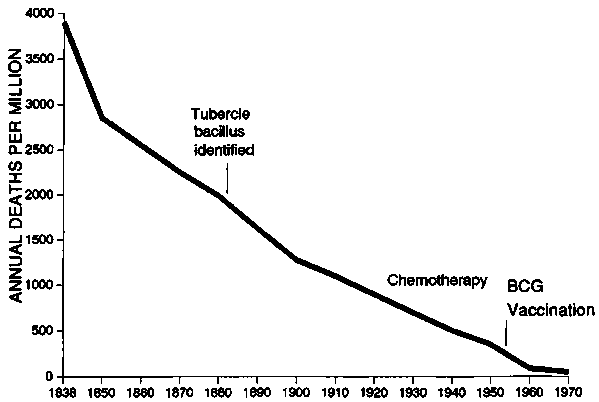
FIG. 8. Fall in deaths from whooping cough among children in England and Wales from 1850 to 1960. Most of the drop occurred before specific treatment or immunization was available. Source: ref. 50
The further declines when specific therapies, such as sulfonamides and antibiotics, became available for these diseases are less significant. However, the effects of modern therapeutic and preventive methods have been dramatic for some diseases. Smallpox was still a scourge throughout most of this century, although the demonstration by Jenner of the first practical vaccination against the disease in 1796 resulted in its virtual elimination in the industrialized countries. The development of a stable freeze-dried vaccine that minimized cold chain requirements plus the use of the bifurcated needle made possible a successful global smallpox eradication programme.
When I was a medical student in 1942-1945, each summer was dreaded because of poliomyelitis epidemics. The numerous cases of death or paralysis from polio seemed a never-ending tragedy. Today thousands in the United States and throughout the world are experiencing the exacerbation of their symptoms of this childhood disease that often return late in life. But there have been no new cases of poliomyelitis in the Western Hemisphere since 1989, and we have the means to eradicate this disease from all of the world. An intensive UNICEF/WHO campaign has now brought the benefits of immunization against diphtheria, tetanus, whooping cough, measles, and poliomyelitis to over 80% of the children of many developing countries [51].
When I left for an internship in the Panama Canal Zone in 1945, a large ward in Rochester General Hospital was filled with women with pelvic inflammatory disease. When I returned a year later, the ward had been closed because penicillin had become available to treat it. Tuberculosis was the leading cause of death in Panama at the time, but the availability of streptomycin and isoniazid soon changed this. Historically, the introduction by Gorgas of sanitary measures that conquered yellow fever made possible the construction of the Panama Canal, where an earlier French effort had failed because of this disease.
More recently, residual treatment of houses with DDT and treatment of cases with chloroquine have reduced or eliminated malaria from populations where it had been devastating. Unfortunately, the rise of mosquitoes resistant to DDT and of parasite strains resistant to chemotherapy is responsible for a global resurgence of this disease. Tuberculosis once seemed on the verge of extinction because of effective chemotherapy, but is now increasing once again due to the development of drug-resistant strains. The resurgence is made worse by the weakened immunity associated with the acquired immunodeficiency syndrome (AIDS) epidemic. Hookworm disease, typhoid fever, and cholera are no longer a threat to the populations of industrialized countries, because of improved environmental sanitation and hygiene.
The classic nutritional deficiency diseases of scurvy, beriberi, and pellagra have also virtually disappeared, except for a recent recurrence in refugee populations. The decline in Japan of the thiamine-deficiency disease beriberi from a death rate of 25 per 100,000 in 1930 to 1 per 100,000 in 1955 was dramatic. In the United States, the niacin-tryptophan-deficiency disease pellagra increased during the depression of the 1930s and then dropped rapidly and disappeared. In neither case was the discovery of the vitamin deficiency responsible for the disease a significant factor in most of the decline. In the McKeown review, the drop in maternal mortality in England, Scotland, and Wales was as striking as the decreases in mortality from infectious diseases in the same period [50]. In recent decades, in country after country, improving nutrition and sanitation have brought rapid declines in infant mortality. The millions of cases of death and disability from these diseases would not have occurred if past social conditions had been different. The same applies to most of the malnutrition and infection that persists today.
We are increasingly recognizing the profound effect of diet on the incidence and severity of chronic degenerative diseases. This is no longer news, but it is a striking example of our capacity to eliminate a great deal of the disability and premature death in older adults.
In the last few decades there has been increasing evidence of the influence of diet on hypertension, cardiovascular disease, type II diabetes, and some forms of cancer [52]. It should be noted that the modern diet is profoundly different from that consumed during the long period of human evolution.
Dietary lipids and heart disease
In the 1950s Ancel Keys made his classic observation, in a number of countries, of a linear relationship between cardiovascular disease mortality and serum cholesterol levels. He also noted a direct relationship between serum cholesterol levels and the amounts of total and saturated fat in the diet. These observations were the forerunner of intensive experimental, epidemiological, and intervention research, which subsequently confirmed them. For example, a 1981 international cooperative study found the same relationship between dietary fat and mean serum cholesterol [53].
The Institute of Nutrition of Central America and Panama-Louisiana State University (INCAP-LSU) Interamerican Atherosclerosis Project in the 1960s graded the atherosclerotic lesions in the aortas and coronary vessels from serial autopsies in large urban public hospitals in 14 countries [54]. In New Orleans and Oslo, lesions began to be clinically significant in young adults, and coronary deaths were increasingly frequent with age. But in Guatemala, Colombia, Chile, Costa Rica, and other developing countries, lesions were not sufficiently severe at any age to be associated with significant coronary heart disease. These results closely paralleled the percentage of dietary calories from fat [55]. Concurrently, the observation of surprisingly advanced atherosclerosis in many young American soldiers killed in Vietnam shocked investigators.
This article cannot possibly review the extensive evidence for the role of diet in coronary heart disease, but this has been done well by Ulbricht and Southgate [56]. There is a consensus among nearly all investigators and expert groups that the cholesterol carried in low-density lipoproteins is the principal culprit and that high-density lipoproteins are protective. When fat supplies less than 25% of dietary calories, coronary heart disease is rare. However, it is principally the content of saturated fats, including trans-fatty acids, that increases serum cholesterol and causes damage when dietary cholesterol is also moderate to high. Contributory factors are hypertension, obesity, and lack of physical activity.
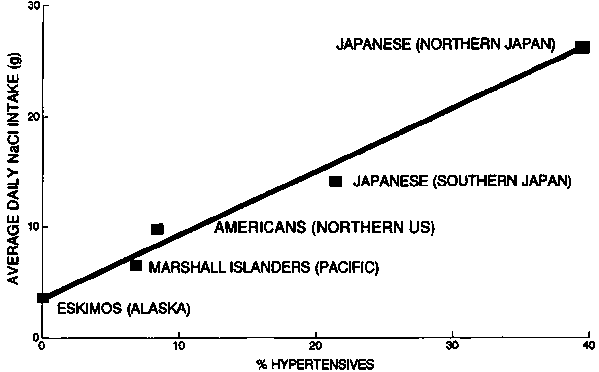
FIG. 9. Correlation of average daily salt (NaCl) intake with prevalence of hypertension in different geographic areas and among different races
Because diets rich in grains, vegetables, and fruits seem to be protective, there is speculation and some evidence that dietary fibre, dietary antioxidants such as vitamins E and C and carotenoids, and other dietary factors are also involved [57]. Moreover, there is quite convincing evidence that coronary lesions can even be reversed by extremely stringent diets combined with other lifestyle changes. The significance of all of this is that disability and death from coronary heart disease are largely preventable.
The same is true for hypertension and stroke. A classic review of hypertension in 27 countries showed a direct relationship with salt consumption [58]. This has been confirmed by the Intersalt Study in 52 centres in 32 countries. About 30% of the population is sensitive to salt intakes that are lower than those in most industrialized country diets [59]. In populations consuming less than 4.5 g of salt daily, an age-related rise in blood pressure is slight or absent, and the frequency of hypertension is uniformly low. As sustained intakes rise progressively above 6 g daily, blood pressure rises with age and hypertension is increasingly frequent. Obesity, saturated fat in the diet, and high alcohol consumption are also important contributors to hypertension [57]. Strokes were the leading cause of death in Japan until they were recently overtaken by heart disease, and they are far less common in the United States. The relatively high rates of hypertension and associated salt consumption in Japan are shown in figure 9.
Osteoporosis
Osteoporosis is another serious degenerative condition, especially in post-menopausal women. Low dietary calcium intakes in childhood result in bones of lower density at puberty that are more susceptible to osteoporosis in later years, as calcium is gradually lost from bones. After years of uncertainty, it is now clear that adequate calcium intake by adults can at least retard the development of this disease [57].
Cancer
The wide variations in cancer rates among different populations provide strong evidence that cancers are greatly influenced by environmental factors. The incidence of stomach cancer is high in South America and in Japan and other parts of Asia, and low in North America and Europe. The low breast cancer rate among Japanese women in Japan was at first ascribed to genetic factors, but the higher rates among ethnic Japanese in California provided evidence to the contrary.
Both experimental and epidemiological studies have pointed to dietary fat as an important determinant of prostate, colon, and breast cancer mortality. Figure 10 indicates a relationship between dietary fat and national rates for breast cancer [60]. There is also a strong relationship between breast cancer in various ethnic groups and their dietary fat intake. Suspected relationships between various diet components and various types of cancer are shown in table 4 [52]. It is estimated that 35% to 40% of cancers in the United States are diet related [57]. Some evidence indicates a preventive role for the antioxidant vitamins E and C and beta carotene, but this is mainly an extrapolation from the beneficial effects of diets supplying these nutrients in abundance. Attention has also been focused on the possible benefits of some of the many biologically active non-nutrient components in green and yellow vegetables, fruits, and legumes. Some of these are listed in table 5.
TABLE 4. Associations between selected dietary components and cancer
|
Site of cancer |
Fat |
Body weight |
Fruits and vegetables |
Alcohol |
Smoked, salted, and pickled foods |
|
Lung |
|
|
- |
|
|
|
Breast |
+ |
+ |
|
± |
|
|
Colon |
++ |
|
- |
|
|
|
Prostate |
++ |
|
|
|
|
|
Bladder |
|
|
- |
|
|
|
Rectum |
+ |
|
- |
+ |
|
|
Endometrium |
|
++ |
|
|
|
|
Oral cavity |
|
|
- |
+ |
|
|
Stomach |
|
|
- |
|
++ |
|
Cervix |
|
|
- |
|
|
|
Esophagus |
|
|
- |
++ |
+ |
Source: ref. 52.TABLE 5. Examples of biologically active compounds in fruits and vegetables with potential anticancer activity
|
Fruits and vegetables |
Compounds |
|
Citrus fruits
|
coumarins |
|
limonene |
|
|
Brassica
|
dithiolthiones |
|
isothiocyanates |
|
|
thiocyanates |
|
|
Soya beans
|
phytoestrogens |
|
saponins |
|
|
Vegetables
|
b-sitosterol |
|
phenols |
|
|
polyphenols |
|
|
Cereals |
inositol hexaphosphate |
In the United States more than 40% of adults are either overweight or frankly obese, and rates are even higher in some European countries. In addition to its important contribution to hypertension and heart disease, obesity is a major determinant of adult-onset diabetes and musculoskeletal disorders. Adolescent obesity is a serious problem in most industrialized countries, particularly because it usually leads to overweight or obesity in adulthood. Unless checked by lower fat and total energy intakes and increased physical activity, obesity will be an even more serious problem in the future in industrialized countries. It is also a growing problem in the more affluent population sectors of developing countries.
Much of the ill health and premature mortality of lower socio-economic groups is traceable to poor maternal nutrition and other negative influences on the mother during pregnancy and lactation.
Iodine deficiency in pregnancy
Iodine deficiency in pregnancy has long been known to result in cases of cretinism, a manifestation in the child of severe iodine deficiency during gestation. The typical cretin has profound mental deficiency, a characteristic appearance, a shuffling gait, shortened stature, spastic dysplasia, and limited hearing. The brain of the developing foetus is supplied with thyroid hormone by the mother during pregnancy, and the foetus is particularly at risk if the mother is deficient in iodine. Probably most of the damage occurs in the second trimester, when there is the most rapid development of the neuropile. At about this time also, the cochlea and auditory neurons are developing most rapidly. This may explain the vulnerability of the auditory apparatus to iodine deficiency.
It is now recognized that, even when cases of cretinism are few in number, they indicate a much larger number of persons who do not have the classic signs of cretinism, but whose linear growth, intellectual capacity, and some other neurological functions are compromised to varying degrees. Strong evidence has come from Ecuador, Peru, China, Central Java, rural Spain, Malawi, and Papua-New Guinea [61]. In each of these studies there were clear differences in both neuromotor function and cognition scores between iodine-poor and iodine-rich populations. The damage to the foetal nervous system caused by iodine deficiency during pregnancy is largely irreversible after birth.
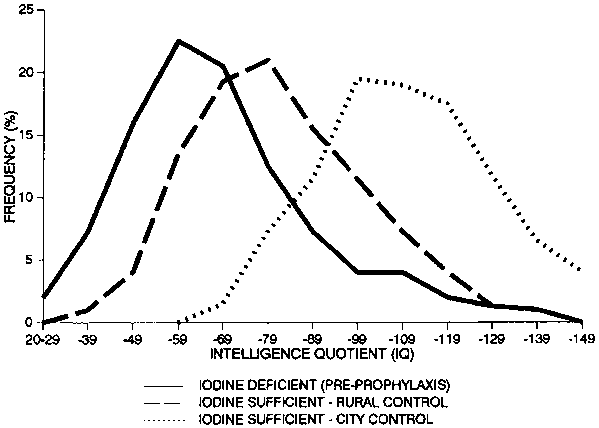
For example, large-scale studies in the People’s Republic of China, Zaire, and Ecuador, comparing villages in which the salt was iodized with control villages, have routinely found differences of approximately 10 percentage points in IQ, depending on the iodine status of the population. This is illustrated in figure 11 for Chinese primary-school children in iodine-deficient and iodine-replete communities [62], and in figure 12 for the offspring of mothers receiving iodine-treated oil, compared with the offspring of control mothers, in Zaire [63]. Similar results have been obtained from comparisons between the offspring of iodine-deficient mothers who received iodinated oil before pregnancy and the offspring of those who did not [64]. The shift in performance was throughout the entire normal distribution curve and not due to a few individuals with very low scores. An example of IQ distribution curves correlated with iodine status is shown in figure 13, for Papua-New Guinea [65].
Approximately one billion people of all ages, in at least 90 countries, are considered to be at risk for iodine deficiency, although cases of endemic goitre are estimated at 200 to 300 million [48]. About six million of these show signs of cretinism, and there are many more with mental retardation or other neurological change.
Folic acid and neural tube defects
Although neural tube defects at birth are relatively rare, the evidence is convincing that the risk of their occurrence can be greatly reduced by a diet adequate in folic acid [66-68].
FIG. 11. IQ scores in students 7 to 13 years of age in two socio-economically similar Chinese communities differing in iodine status. Source: ref. 62
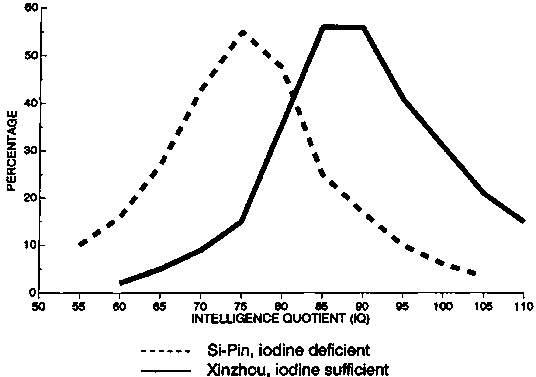
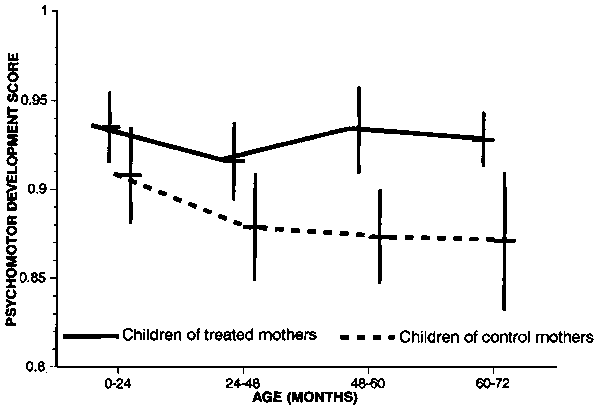
FIG. 12. Effect of treating mothers with iodized oil on psychomotor development scores of children in Zaire. Source: ref. 63
Other maternal behaviours and foetal outcome
A variety of other maternal behaviours can damage the foetus. Excessive alcohol consumption during pregnancy can result in foetal alcohol syndrome, characterized by a pattern of physical and behavioural abnormalities. Narcotic use during this time can damage and addict the foetus. An increasing number of common medicines taken by pregnant women can cross the placental barrier. When this occurs during the period of organogenesis, profound physical and mental handicaps can result [69]. Drugs that are vitamin antagonists are particularly risky.
One of the most surprising recent developments is the finding that poor nutrition during foetal development and infancy influences the occurrence of chronic diseases in adulthood.
FIG. 13. IQ scores in three populations in Papua-New Guinea differing in iodine status. Source: ref. 65
Foetal growth restriction due to maternal malnutrition and other adverse environmental factors leads to a small-for-gestation baby. Today this is common throughout the developing world; but in the past, in today’s industrialized countries, it has been characteristic of lower socio-economic groups. D. J. P. Barker, Professor of Clinical Epidemiology at the University of Southampton, and his colleagues have identified groups and individuals in England who suffered foetal growth retardation and/or stunting at 12 months of age, and determined their health as older adults [70, 71].
Cardiovascular disease and hypertension
One of the first relationships to be evident from the Barker studies was that of birthweight and weight at one year of age with cardiovascular disease among men born in the early decades of this century. The progressive decrease in adult deaths from cardiovascular disease with increased weight at one year of age for men born between 1911 and 1930 in Hertfordshire, England, is shown in table 6 [72]. The same relationship was observed between either head circumference or ponderal index at birth, and mortality rate from cardiovascular disease, in a population born in Preston, England, between 1935 and 1943 [73].
Law et al. [74] conducted a follow-up study of the populations of Preston and Hertfordshire, England, and a national sample in Britain, to determine whether the relationship between high blood pressure and low birthweight is initiated in utero or during infancy. The sample included nearly 2,000 children aged 0 to 10 years, 3,200 men and women aged 36 to 45 years, almost 500 men and women aged 46 to 54 years, and about 1,200 men and women aged 59 to 71 years. The results indicated that essential hypertension is influenced by foetal nutrition and is amplified by dietary and other factors from infancy to old age.
TABLE 6. Standardized mortality ratios for ischaemic heart disease according to weight at one year of age in 6,500 men born between 1911 and 1930 in England
|
1-yr weight (lb)
|
Mortality ratio (no. of deaths) |
|
|
Ischaemic heart disease |
All non-circulatory disease |
|
|
£18 |
100 (36) |
74 (39) |
|
19-20 |
84 (90) |
99 (157) |
|
21-22 |
92 (180) |
74 (215) |
|
23-24 |
70 (109) |
67 (155) |
|
25-26 |
55 (44) |
84 (99) |
|
³27 |
34 (10) |
72 (31) |
|
All |
78 (469) |
78 (696) |
Source: ref. 72.The Preston, England, study of 449 women born in Lancashire, England, from 1935 to 1943 included their placental weight at birth [75]. High placental weights are associated with iron deficiency in the mother. The blood pressure of these women around age 50 was strongly predicted by a comparison of their placental and body weights at birth. The worst combination was a small body weight and a large placenta. There is a report from the Gambia [76] that the blood pressure of children at eight years of age is inversely proportional to their mothers’ weight gain in the last trimester of pregnancy.
TABLE 7. Mean two-hour plasma glucose and insulin concentrations, and percentages of men and women with impaired glucose tolerance (IGT) or non-insulin-dependent diabetes mellitus, according to birthweight
|
Birthweight |
2-h glucose mmol/L |
2-h insulin pmol/L |
% with IGT or newly diagnosed non-insulin-dependent
diabetes |
Odds ratio adjusted for BMI |
|
£2.50 |
6.2 (30) |
207.5 (30) |
27 (30) |
6.4 (1.8 to 23) |
|
2.51-2.95 |
5.9 (55) |
207 (53) |
18 (55) |
3.3 (1.0 to 11) |
|
2.96-3.41 |
5.6 (99) |
147.4 (97) |
11 (99) |
1.9 (0.6 to 5.9) |
|
>3.41 |
5.3 (82) |
139.9 (80) |
6 (82) |
1.0 |
|
Total |
5.6 (266) |
161.7 (260) |
13 (266) |
X2 for trend = 9.9 |
Source: adapted from ref. 77.
FIG. 14. Proportions of 64-year-old men with impaired glucose tolerance or diabetes according to weight (to nearest pound) at one year. Sample was 370 men in Hertfordshire, England. Source: ref. 78

Late-onset diabetes
The Barker group has also explored the correlation between adult-onset diabetes and weight at birth and at one year of age [77]. Table 7 shows birthweight linked to glucose intolerance and non-insulin-dependent diabetes for a Preston population at 46 to 54 years of age [77]. The relationship between weight at one year and glucose tolerance for the Hertfordshire population, shown in figure 14, is equally striking [78]. These observations help to explain why formerly undernourished populations develop such high diabetes rates with rising affluence.
These findings do not mean that the environment in adult life is unimportant for chronic degenerative diseases (it is still very important), but they do help to explain why the known adult risk factors, demonstrable in populations, are poor predictors of cardiovascular disease in individuals. These findings of the potency of combined early and later risk factors strengthen the conclusion that the bulk of cardiovascular disease and adult-onset diabetes is due to environmental, rather than genetic, causes. They also suggest that an increase in meat, fat, and calorie consumption with rising affluence in formerly poorly nourished populations is particularly hazardous, because individuals born of poorly nourished mothers are more susceptible.
Autoimmune thyroid disease
In the Hertfordshire population born from 1911 onwards, sera were available for more than 300 women, now aged 60 to 71, that could be measured for antithyroglobulin antibody and thyroid peroxidase antibody [79]. Nearly 6% of these women had already been diagnosed as being hypothyroid. A strong direct relationship was also found between birth weight and the percentage of women with the antithyroglobulin antibody.
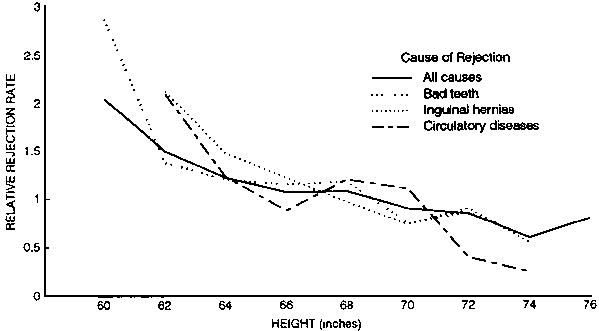
FIG. 15. Rejection rates in 4,245 men aged 23-49 examined for the Union Army (correlated to height)
The economic historian Robert Fogel, winner of the 1993 Nobel Prize in economics, has initiated a fascinating study of the life histories of American Civil War veterans, in which I have been involved. We have access to the medical records and subsequent periodic medical evaluations for the award of pensions for nearly 30,000 veterans. We are finding that the prevalences of chronic diseases at older ages were much higher among these individuals than among US veterans of World War II who reached age 65 and over in the 1980s. For example, musculoskeletal diseases were 1.6 times more prevalent, cardiovascular disease 2.9 times more prevalent, and digestive diseases 4.7 times more prevalent among veterans 65 or more in 1910 than in veterans in the same age range in the 1980s. The average body size increased by more than 20% among men 50 to 64 between the 1880s and the 1990s [80]. Other studies suggest that in some populations in Europe maintaining the same genetic potential, body size has increased by more than 50% in the past 10 generations.
In studying health outcomes in populations, height is a surrogate for early nutrition and related adverse socio-economic factors. As illustrated in figure 15, we found a strong relationship between height and rejection rate for bad teeth, hernias, and circulatory diseases among recruits whose height per se did not exclude them from service [80]. The survival of Civil War veterans with cardiovascular disease at age 60 was also influenced by height [80]. This phenomenon has been explored by Waaler in a Norwegian population that showed a striking influence of body height on the relative mortality rates among men aged 40 to 59 between 1963 and 1979 [81].
We are also learning how much lifestyle affects the ageing process. Two decades ago, the scientific literature was filled with gloomy studies of the ageing process, reporting inexorable physical and mental deterioration. Nursing-home studies reinforced this stereotype. More recently, studies of healthy free-living elderly have shown extraordinary variation in the functional changes with age, and indicate that the extent of the changes depends to a remarkable degree on lifestyle and the social environment. For example, in the United States the great majority of persons over 70 are still leading active independent lives.
Studies at the Center for Nutrition and Aging at Tufts University in Boston confirm not only that the deterioration of lean body mass and muscle strength with ageing can be halted by appropriate physical activities and a good diet, but also that it can be reversed to a significant degree [82]. Similarly, people who remain socially and intellectually active age quite differently from those who become sedentary and lack stimulus. Much of what has been considered the normal process of ageing can be strongly influenced by environmental factors, including nutrition and the social environment.
Although nutrition is important at all ages, it is only one of the environmental factors determining the expression of our genetic potential. Of other lifestyle factors that are preventable contributors to ill health, tobacco smoking is the most important in many societies. As a major factor in the occurrence of emphysema, respiratory infections, lung cancer, and coronary heart disease, smoking enormously increases the burden of ill health on individuals and societies, as well as the number of premature deaths. It affects not only the smoker but also those persons in close proximity.
Seat belts, airbags, and all other types of safety measures reduce death and disability from accidents. Driving while intoxicated is behaviour with frequent negative consequences. Most HIV infection is the result of risky behaviours or failure to maintain the safety of blood products.
Stressful behaviours and inability to cope with them are also contributory factors to the development of hypertension and heart disease and may, through an effect on immune mechanisms, affect susceptibility to cancer.
The physical environment, as well as the biological and social environments, influences health. Another essay could be written about the adverse effects of environmental pesticides, toxicants, and other environmental pollution of all kinds. In many of the large cities in the developing world, the air is so polluted from automobiles, trucks, buses, and industry that children are growing up with levels of lead known to impair cognitive development, and persons of all ages have a serious increase in respiratory infections. Moreover, the situation is worsening in most of these cities as they grow, and many millions of persons are affected.
Unsafe water supplies, unsanitary disposal of human wastes, and poor food-handling practices that spread enteric diseases are additional contributors to ill health. The significant characteristic of these adverse environmental factors influencing human health is that they are the consequences of human activities and can largely be prevented by social changes.
Knowledge of behaviours that can promote health relies on experimental, clinical, and epidemiological research and intervention trials. Health may also be improved by applying research advances not contemplated in nature. One of the earliest examples is vaccination against smallpox. The identification of insulin for the treatment of diabetes and of thyroid hormone for the treatment of hypothyroidism and Graves’ disease are other early examples.
The risk of tetanus, diphtheria, measles, and whooping cough can now be eliminated by effective immunization programmes. There have been no cases of poliomyelitis in the Western Hemisphere since 1989, as a result of strong immunization efforts. The discovery of sulfonamides and antibiotics not only revolutionized infectious-disease therapy but also reduced the risk of acquiring many infections. As another example, estrogen replacement therapy in women after menopause reduces the risk of osteoporosis.
For some time now the examination of cells obtained by amniocentesis has made it possible to detect a number of devastating congenital abnormalities in time to allow for possible termination of the pregnancy. The next major advance will be the capacity to treat patients afflicted with genetic diseases by inserting foreign genes into their cells, even in utero. A steady stream of further health advances can be anticipated from current biotechnology research.
Even the food industry is improving on nature by producing an increasing variety of palatable low-fat and fat-free products. Nutrient enrichment of common foods is also an example of technology improving on nature.
Although every life begins with an individual genetic potential, this potential frequently begins to be compromised in utero when the mother is affected by malnutrition, infection, or other stress factors. Iodine deficiency in the first and second trimester can lead to permanent neurological damage to the child. Low weight for duration of gestation predicts not only poorer health in early childhood but also more problems of chronic degenerative diseases in adults as they age. In underprivileged children, the synergism of malnutrition and infection commonly impairs growth and development and may lead to both physical and mental stunting. Throughout life, the quality of the diet influences the occurrence of both acute and chronic diseases, including hypertension, heart disease, type II diabetes, and some of the most serious forms of cancer.
Other adverse lifestyle factors include obesity, smoking, inactivity, stress, and exposure to environmental contaminants, pollutants, and toxicants. They contribute to health outcomes that fall far short of an individual’s genetic potential. Personal lifestyle is a major determinant of health and function of the elderly. Deaths from trauma and violence further cut short what genetically should be long and healthy lives.
Hereditary diseases and metabolic disorders seriously affect some individuals, but most ill health and premature death is preventable. This is the new paradigm! It calls for a multidisciplinary effort in which the role of the social scientist is as important as that of the health scientist in promoting health, and both have more to offer in this effort than those exclusively concerned with curative medicine. Moreover, success would eliminate a great deal of the current economic costs of treating disease and disability, as well as their social costs to individuals and society.
1. Scrimshaw NS, Taylor CE, Gordon JE. Interactions of nutrition and infection. Am J Med Sci 1959; 237(3): 367403.
2. Waterlow JC, Schürch B. eds. Causes and mechanisms of linear growth retardation (Proceedings of an IDECG workshop held in London, 15-18 January 1993). Eur J Clin Nutr 1994; 48(S1).
3. Mönckeberg F. Effect of early marasmic malnutrition on subsequent physical and psychological development. In: Scrimshaw NS, Gordon JE, eds. Malnutrition, learning and behavior. Cambridge, Mass, USA: MIT Press, 1968: 269-77.
4. Champakan S. Srikantia SG, Gopalan C. Kwashiorkor and mental development. Am J Clin Nutr 1968; 21: 844-855.
5. Grantham-McGregor SM, Powell CA, Walker SP, Himes JG. Nutritional supplementation, psychological stimulation and development of stunted children: the Jamaican study. Lancet 1991; 338: 1-5.
6. Gomez SF, Velasco AJ, Ramos GR, Cravioto J. Frenk S. Studies on malnourished children. XVII: Psychological manifestations. Med Hosp Inf (Mex) Bol 1954; II: 631-41.
7. Pollitt E, Granoff D. Mental and motor development of Peruvian children treated for severe malnutrition. Rev Interam Psi 1967; 1: 93-102.
8. Yatkin US, McClaren DS. The behavioral development of infants recovering from severe malnutrition. J Ment Def Res 1970; 14: 25-32.
9. Gerber M, Dean RFA. The psychological changes accompanying kwashiorkor. In: Wickert FR, ed. Readings in African psychology from French language sources. East Lansing, Mich, USA: Michigan State University Press, 1967: 295-311.
10. Cravioto J. DeLicardie ER. Neurointegrative development and intelligence in children rehabilitated from severe malnutrition. In: Prescott JW, Read MS, Coursin D, eds. Brain function and malnutrition: neuropsychological methods of assessment. New York: John Wiley, 1975: 53-72.
11. Mönckeberg F. The effect of malnutrition on physical growth and brain development. In: Prescott JW, Read MS, Coursin D, eds. Brain function and malnutrition: neuropsychological methods of assessment. New York: John Wiley, 1975: 15-52.
12. Cravioto J. DeLicardie ER, Birch HG. Nutrition, growth and neurointegrative development: an experimental and ecologic study. Pediatrics 1966; 38 (suppl 2, part 2): 319-72.
13. Cravioto J. DeLicardie ER. Intersensory development of school aged children. In: Scrimshaw NS, Gordon JE, eds. Malnutrition, learning and behavior. Cambridge, Mass, USA: MIT Press, 1968: 252-69.
14. Mora JO, Herrera MG, Sellers SG, Ortiz N. Nutrition, social environment and cognitive performance of disadvantaged Colombian children at three years. Nutrition in health and disease and international development (Symposia from the XII International Congress of Nutrition). New York: Alan R. Liss, 1981: 403-30.
15. Sigman M, Neumann C, Jansen AA, Bwibo N. Cognitive abilities of Kenyan children in relation to nutrition, family characteristics and education. Child Dev 1989; 60: 1463-74.
16. Klein RE, Irwin M, Engel PL, Yarbrough C. Malnutrition and mental development in Guatemala: an applied cross-cultural research study. In: Warren N. ed. Advances in cross-cultural psychology. New York: Academic Press, 1977: 92-121.
17. Chavez A, Martinez C. Growing up in a developing community. Mexico City: National Nutrition Institute, 1982.
18. Martorell R. Enhancing human potential through improved nutrition in early childhood. Nutr Today 1993; Jan/Feb: 6-13.
19. Pollitt E, Gorman KS, Engle PL, Martorell R. Rivera J. Early supplementary feeding and cognition. Monographs of the Society for Research in Child Development. Chicago, Ill, USA: University of Chicago Press, 1993; 58(7)
20. Chavez A, Martinez C, Soberanes B. The effect of malnutrition on human development: a 24-year study of well-nourished and malnourished children living in a poor Mexican village. In: Scrimshaw NS, ed. Community-based longitudinal nutrition and health studies: classical examples from Guatemala, Haiti and Mexico. Boston, Mass, USA: International Nutrition Foundation for Developing Countries, 1995: 79-124.
21. McKay H, Sinisterra L, McKay A, Gomez H, Lloreda P. Improving cognitive ability in chronically deprived children. Science 1978; 200: 2780-7.
22. Lutter CK, Mora JO, Habicht JP, Rasmussen KM, Robson DS, Sellers SG, Super CM, Herrera MG. Nutritional supplementation: effects on child stunting because of diarrhea. Am J Clin Nutr 1989; 50: 1-8.
23. Pollitt E, Oh S-Y. Early supplementary feeding, child development, and health policy. Food Nutr Bull 1994; 15: 208-14.
24. Shetty PS, James WPT. Body mass index, a measure of chronic energy deficiency in adults. FAO Food and Nutrition Paper 56. Rome: Food and Agriculture Organization, 1994.
25. James WPT, Ralph A, eds. The functional significance of low bodymass index. Eur J Clin Nutr 1992; 48 (suppl 3)
26. Satyanarayana K, Rao SS, Radhiah G. Reddy V. Body mass index and mortality rates. Nutr News (Hyderabad, India: National Institute of Nutrition) 1991; 12.
27. James WPT, Ferro-Luzzi A, Waterlow JC. Definition of chronic energy deficiency in adults. Report of a working party of the International Dietary Energy Consultative Group. Eur J Clin Nutr 1988; 42: 969-81.
28. Naidu AN, Rao NP. Body mass index: a measure of the nutritional status in Indian populations. Eur J Clin Nutr 1994; 48: S131-40.
29. Ending hidden hunger: a policy conference on micro-nutrient malnutrition, October 10-12, 1991, Montreal. Atlanta, Ga, USA: Task Force for Child Survival and Development, 1992.
30. Scrimshaw NS. Functional significance of iron deficiency: an overview. In: Enwonwu CO, ed. Functional significance of iron deficiency. Annual Nutrition Workshop Series, Vol. III. Nashville, Tenn, USA: Meharry Medical College, Center for Nutrition, 1990: 1-13.
31. Walter T. Infancy: mental and motor development. Am J Clin Nutr 1989; 50(suppl 3): 655-66.
32. Lozoff B. Methodologic issues in studying behavioral effects of infant iron-deficiency anemia. Am J Clin Nutr 1989; 50: 641-54.
33. Lozoff B. Brittenham GM, Viteri FE, Wolf AW, Urrutia JJ. The effects of short-term oral iron therapy on developmental deficits in iron-deficient infants. J Pediatr 1982; 100: 351-7.
34. Sinisterra L, McKay A, McKay H. Gomez H. Korgi J. Response of malnourished preschool children to multidisciplinary intervention. In: Brozek J. ed. Malnutrition and human behavior: experimental, clinical and community studies. New York: Van Nostrand Reinhold, 1985: 317-26.
35. Pollitt E, Hathirat P. Kotchabhakdi NJ, Missell L, Valyasevi A. Iron deficiency and educational achievement in Thailand. Am J Clin Nutr 1989; 50 (suppl 3): 687-97.
36. Soewando S. Husaini M, Pollitt E. Effect of iron deficiency on attention and learning processes in preschool children: Bandung, Indonesia. Am J Clin Nutr 1989; 50 (suppl 3): 667-74.
37. Idjradinata P. Pollitt E. Reversal of developmental delays in iron-deficient anaemic infants treated with iron. Lancet 1993; 341: 1-4.
38. Soemantri AG. Preliminary findings on iron supplementation and learning achievement of rural Indonesian children. Am J Clin Nutr 1989; 50 (suppl 3): 698-702.
39. Pollitt E, Soemantri AG, Yunis F. Scrimshaw NS. Cognitive effects of iron-deficiency anemia (letter). Lancet 1985; 1 (8421): 158.
40. Seshadri A, Gopaldas T. Impact of iron supplementation on cognitive functions in preschool and school-aged children: the Indian experience. Am J Clin Nutr 1989; 50 (suppl 3): 675-86.
41. Oski FA, Honig AS. The effects of therapy on the development scores of iron-deficient infants. J Pediatr 1978; 92: 21-5.
42. Webb T. Oski F. Iron deficiency anemia and scholastic achievement in young adolescents. J Pediatr 1973; 82: 827-30.
43. Pollitt E, Lewis N. Leibel RL, Greenfield DB. Iron deficiency and play behavior in preschool children. In: Garry PJ, ed. Human nutrition. Washington, DC: American Association for Clinical Chemistry, 1981: 290-301.
44. Pollitt E, Viteri F. Saco-Pollitt C, Leibel RL. Behavioral effects of iron deficiency anemia in children. In: Pollitt E, Leibel RL, eds. Iron deficiency: brain biochemistry and behavior. New York: Raven Press, 1982: 195-208.
45. Lozoff B. Jimenez E, Wolf AW. Long term developmental outcome of infants with iron deficiency. N Engl J Med 1991; 325: 687-95.
46. Beard JL, Feagans L, Frobose C. Cognitive dysfunction in iron deficient adolescents (abstract). FASEB J 1995; 9(4): A975.
47. Bruner AB, Joffe A, Duggan AK, Casella JF, Barsky SH. Randomised study of cognitive effects of iron supplementation in non-anaemic iron-deficient adolescent girls. Lancet 1996; 348: 992-6.
48. World Health Organization. Global nutrition status update 1989. Geneva: World Health Organization Publications, 1989.
49. Beaton GH, Martorell R. L’Abbé KA, Edmonston B. McCabe G. Ross AC, Harvey B. Effectiveness of vitamin A supplementation in the control of young child morbidity and mortality in developing countries. Toronto: University of Toronto Press, 1993.
50. McKeown T. The modern rise of population. New York: Academic Press, 1976.
51. UNICEF. The state of the world’s children 1985. Oxford, UK: Oxford University Press, 1984.
52. World Health Organization. Diet, nutrition, and the prevention of chronic diseases. Technical Report Series 797. Geneva: World Health Organization, 1990.
53. Berkson DM, Stamler J. Epidemiology of the killer chronic diseases. Curr Concepts Nutr 1981; 10: 17-55.
54. Tejada C, Strong JP, Montegegro MR, Restrepo C, Soberg LA. Distribution of coronary and aortic atherosclerosis by geographic location, race and sex. Lab Invest 1968; 18: 509-26.
55. Scrimshaw NS, Guzman MA. Diet and atherosclerosis. Lab Invest 1968; 18: 623-8.
56. Ulbricht TLV, Southgate DAT. Coronary heart disease: seven dietary factors. Lancet 1991; 338: 985-92.
57. National Research Council. Diet and health: implications for reducing chronic disease risk. Washington, DC: National Academy of Sciences Press, 1989.
58. Dahl LK, Heine M, Tassinari L. Effects of chronic excess salt ingestion: evidence that genetic factors play an important role in susceptibility to experimental hypertension. J Exp Med 1962; 115: 1173-90.
59. Intersalt Cooperative Research Group. Intersalt: an international study of electrolyte excretion and blood pressure. Result for a 24 hour urinary sodium and potassium excretion. BMJ 1988; 197: 319-28.
60. Carroll KK, Khor HT. Dietary fat in relation to tumor-igenesis. Prog Biochem Pharmacol 1975; 10: 308-53.
61. Stanbury JB, ed. The damaged brain of iodine deficiency. Elmsford, NY, USA: Cognitive Communications Corp., 1994.
62. Ma T, Wang D, Chen ZP. Mental retardation other than typical cretinism in IDD endemias in China. In: Stanbury JB, ed. The damaged brain of iodine deficiency. Elmsford, NY, USA: Cognitive Communications Corp., 1994: 265-72.
63. Vanderpas J. Thilly CH. Endemic neonatal, infantile, and juvenile hypothyroidism in Ubangi, Northern Zaire: clinical consequences and prevention. In: Stanbury JB, ed. The damaged brain of iodine deficiency. Elmsford, NY, USA: Cognitive Communications Corp., 1994: 209-24.
64. Bautista A, Barker PA, Dunn JT, Sanchez M. The effects of oral iodized oil on intelligence, thyroid status and somatic growth in school age children from an area of endemic goiter. Am J Clin Nutr 1982; 35: 127-34
65. Boyages SC. The damaged brain of iodine deficiency: evidence for a continuum of effect on the population at risk. In: Stanbury JB, ed. The damaged brain of iodine deficiency. Elmsford, NY, USA: Cognitive Communications Corp., 1994: 251-8.
66. MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet 1991; 338: 131-54.
67. VNIS. Information sheet on neural tube defects. Vitamin Nutrition Information Service, August 1991. Nutley, NJ, USA: Hoffmann-La Roche, 1991.
68. Altman LK. Study finds a vitamin reduces birth defects. New York Times, Friday, 19 July 1991.
69. Mills JL. Protecting the embryo from X-rated drugs. N Engl J Med 1995; 333: 124-5.
70. Barker DJP, ed. Fetal and infant origins of adult diseases. London: BMJ Publishing Group, 1992.
71. Barker DJP. Mothers, babies, and disease in later life. London: BMJ Publishing Group, 1994.
72. Barker DJP. The effect of nutrition of the fetus and neonate on cardiovascular disease in adult life (Symposium on Impact of Diet on Critical Events in Development). Proc Nutr Soc 1992; 51: 135-44.
73. Barker DP, Godfrey KM, Osmond C, Bull A. The relation of fetal length, ponderal index and head circumference to blood pressure and the risk of hypertension in adult life. Perinatal Epidemiol 1992; 6: 35-44.
74. Law CM, Barker DJP, Bull AR, Osmond C. Maternal and fetal influences on blood pressure. Arch Dis Child 1991; 66: 1291-5.
75. Barker DJP, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk for hypertension in adult life. BMJ 1990; 301: 259-62.
76. Margetts BM, Rowland MGM, Foord FA, Cruddas AM, Cole TJ, Barker DJP. The relation of maternal weight to the blood pressures of Gambian children. Int J Epidemiol 1991; 20: 938-43.
77. Phipps IK, Barker DJP, Hales CN, Fall CHD, Osmond C, Clark PMS. Fetal growth and impaired glucose tolerance in men and women. Diabetologia 1993; 36: 225-8.
78. Hales CN, Barker DJP, Clark PMS, Cox LJ, Fall C, Osmond C, Winter PD. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 1991; 303: 1019-22.
79. Phillips DIW, Barker DJP, Osmond C. Infant feeding, fetal growth and adult thyroid function. Acta Endocrinol 1993; 129: 134-8.
80. Fogel RW. Economic growth, population theory, and physiology: the bearing of long-term processes on the making of economic policy. Am Econ Rev 1994; 84: 369-95.
81. Waaler HT. Height, weight and mortality: the Norwegian experience. Acta Med Scand 1984; 679: 1-56.
82. Evans WA, Rosenberg IH. Biomarkers: the 10 determinants of aging you can control. New York: Simon and Schuster, 1991.