According to what we know
about the regulation of amino acid oxidation, we can expect that
minimum rates of oxidation should occur when intake is low, and
when tissue levels are low, as is the case in subjects on a
protein-free diet, for example when the ONL are being measured.
In the adult, in the absence of growth and discounting skin, hair and secretions, amino acids are required as precursors for various metabolites, neurotransmitters, hormones, cofactors, and the like. In addition, as discussed by REEDS (1990), amino acids are also required to replace those lost from the terminal ileum into the large bowel, where amino nitrogen is reabsorbed as ammonia, but where much of the IAA carbon skeletons are lost during bacterial fermentation.
On a protein-free diet, the source of amino acids for these needs is tissue protein which will be mobilised, releasing a mixture of amino acids (Figure 11). Those which serve some metabolic role will be used and eventually transformed to a nitrogenous end-product; those which are not, will be oxidised directly, since the now unbalanced mixture cannot be reutilised for protein synthesis. If the relative pool sizes of the free amino acids is tightly controlled by the oxidative pathways, then the overall rate of N excretion will be determined by the need for the rate-limiting amino acid, the IAA with the highest ratio of metabolic need to concentration in protein. For all other amino acids, their overall oxidative loss will be in excess of their metabolic need.

On a protein-free diet, mobilisation of body protein is assumed to occur to provide specific amino acids to replace those lost through oxidative losses in the lower gut, and through various metabolic pathways. Other amino acids released serve no particular need and will be oxidised since they cannot be reutilised for protein synthesis. Thus, the overall pattern of losses will reflect tissue protein composition rather than the pattern of actual metabolic needs.
In fact, it is an easy task to calculate the rate of loss of body IAAs which gives rise to the ONL, assuming they derive from tissue protein, and assuming this protein has the composition of beef muscle. MILLWARD and RIVERS (1988) defined these losses as the obligatory oxidative losses (OOL). The problem is to determine which is the rate-limiting amino acid. There are two approaches to this particular problem. The first approach, adopted by MILLWARD and RIVERS (1988), involved a comparison of the magnitude of the OOL with those of the (FAO/WHO/UNU, 1985) adult requirement values for IAA in order to test the hypothesis that the Rose requirement values, which form the basis of the current adult values, were likely to be similar to minimum values (Rmin). It was assumed that the excess of dietary non-essential nitrogen and energy in the original balance studies would have depressed oxidative losses. If this was the case, then for the rate-limiting amino acid the magnitude of its OOL should be similar to that in the Rose pattern, while for all other amino acids the limiting OOL should be greater. In fact, the comparison indicated that the value for the S-amino acids were similar in the two patterns, suggesting that the S-amino acids may be the rate-limiting amino acids driving the ONL. However, YOUNG et al. (1989) rightly point out that there are several reasons why the Rose values may be inaccurate. If this is the case, then it is not justifiable to compare the magnitude of the OOL with the Rose requirement values to identify the rate-limiting indispensible amino acid.
An alternative approach is to look at the experimental evidence from selective amino acid supplementation or depletion studies. MILLWARD and RIVERS (1988) reported that, in several species, the addition of methionine to a protein-free diet reduced nitrogen excretion. The most extensive studies of this kind in adult rats are those of Yoshida (see YOKOGOSHI and YOSHIDA, 1981), who evaluted the nitrogen balance responses to supplementing rats on protein-free diets, and on rice and wheat-protein diets with individual amino acids. They clearly showed that the pattern of the supplementation neccessary for nitrogen balance was dominated by threonine and methionine with relatively little need for leucine and lysine FULLER et al. (1989) held pigs at N equilibrium with a low-protein diet and examined the impact of depletion of individual IAA on N balance. The removal of the sulphur amino acids from the diets induced a negative nitrogen balance almost as great as a protein-free diet, and greater than the removal of any other single amino acid. This indicated that, for the pig, the S-amino acids may be the rate-limiting amino acids driving the ONL, with threonine second limiting, and with lysine and leucine appearing to be of lesser metabolic importance.
What this means is that Rmin for the S-amino acids may be close to the S-amino acid content of the OOL, but for all other amino acids, particularly leucine and lysine Rmin is less. In other words, the pattern of the OOL must be quite different from the minimum requirement pattern. There is nothing remarkable about this since there is no a priori reason why the pattern of minimum requirements, which are metabolic needs, should be the same as that of tissue protein. In particular lysine and leucine, which are of major importance for protein accretion due to their high concentrations in tissue protein, appear to have less importance in the context of metabolic needs.
This difference between maintenance and growth needs was clearly confirmed by FULLER et al. (1989) in his pig experiments (Figure 12). The pattern of the obligatory oxidative losses, which MILLWARD and RIVERS (1988) calculated from the pattern of beef muscle, bears a closer resemblance to the growth rather than the maintenance requirement. In particular leucine and lysine dominate the growth requirement and the OOL pattern, whilst the sulphur amino acids and threonine dominate the maintenance requirements. REEDS (1990) has argued that, in the latter case, this reflects the predominance of the sulphur amino acids and threonine in the amino acid mixture lost from the terminal ileum into the large bowel.
Thus, the pattern of Rmin does appear to be different from the pattern of IAAs in tissues, and the current requirement values for adults could be close to the values for the minimum requirements (i.e., where Lr is close to Lr min). They were obtained in balance studies not with real proteins but with mixtures of amino acids containing very low levels of the 8 IAAs with excess non-essential nitrogen (NEN). However, as already indicated, YOUNG et al. (1989) believe that little attention should be paid to these values because of the inadequacy of the N-balance studies used by Rose. This criticism however cannot be levelled at FULLER et al. (1989), who also used this type of amino acid mixture in their pig experiments. As reviewed elsewhere (MILLWARD and RIVERS, 1988), there is ample evidence in the literature that NEN suppresses IAA oxidation.
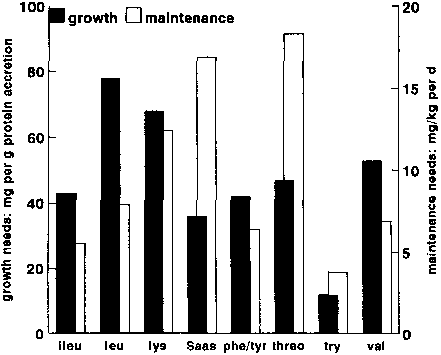
Maintenance requirements are calculated for a 70 kg pig, while growth requirements are for the accretion of I g protein (from FULLER et al., 1989).
It should
be clear from the above that, in the pig, the minimum maintenance
requirement pattern is quite different from the patterns of IAAs
in tissue proteins (the pattern of the OOL). This evidence casts
doubt on using values for the OOL as the basis for new
requirement pattern in humans, as suggested by YOUNG et al.
(1989).
YOUNG et al. (1989)
support their new scoring pattern with stable isotope studies
which point to considerable needs for leucine and lysine However,
I have great difficulties with these studies, both in terms of
their design, and in terms of the technical problems (MILLWARD
and RIVERS, 1988). One particular technical problem, not
previously considered and relating to all stable isotope studies,
is the magnitude of the tracer.
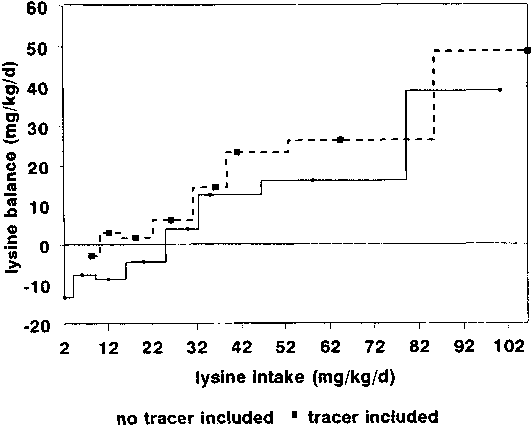
Consider
lysine studies, which are the most important in practical terms.
In these studies, lysine oxidation was measured with 13C
lysine in individuals as lysine intakes were lowered and a
24-hour lysine balance was calculated from the measured fed-state
oxidation rate and estimated fasted losses. The studies appeared
to show that intakes of about 20 mg/kg were required for a
positive balance. In fact these results were obtained by
calculations which largely ignored the impact of the tracer. If,
in contrast, the tracer had been included as input, in the way in
which Young and colleagues have calculated subsequent balances
(PELLETIER et al., 1991), then a much lower balance point
would have been obtained (Figure 13). Because of this,
these results in my opinion do not offer experimental support for
the high lysine requirement which YOUNG et al. (1989) are
proposing.
The consequence of specific
human needs for IAAs should be variable utilisation of dietary
protein sources according to their IAA content. However, in
contrast to studies in laboratory animals, in which it is easy to
demonstrate differences in the biological value of proteins in
relation to their amino acid content and chemical score, in
humans this is extraordinarily difficult (MILLWARD) et al.,
1989).
While differences between protein sources have been reported in N-balance studies in young adults (e.g., biological values of 0.27 for wheat compared with 0.51 for beef; YOUNG et al., 1975), when the calculated bv of several proteins, measured in separate studies, is examined together, the differences between wheat gluten, and other proteins and mixed diets are much less apparent. This is because of the lack of reproducibility between studies with the same protein.
Within individual studies, inter-individual variability is very marked, with biological values often associated with CVs of 15-20% (e.g., YOUNG et al., 1973), and even 50% (YOUNG et al., 1975). The combination of within-study varability and poor reproducibility between trials means that statistical analysis of the data is well nigh impossible. RAND et al., (1981) calculated the size of the experimental groups necessary to provide significant differences in biological value between proteins with the variability observed in the balance trials done at MIT. They showed that, unless biological values differ by the order of 50%, significant differences cannot be demonstrated without unrealistic numbers of subjects (e.g., 21 subjects needed to discriminate between proteins which differ in their bv by 15% with a beta error of 50%). To reduce the error to a more acceptable 10%, 54 subjects would be needed, and such trials are not feasible.
So it is not always easy in human studies to demonstrate differences between proteins. However, when we can, as with wheat gluten for example, we have to be clear what such results mean. With wheat gluten we assume from animal studies that it is lysine-deficient, but we can only prove that with lysine supplementation studies.
In fact, lysine supplementation studies have not in general demonstrated marked improvements of wheat gluten utilisation (VAGHEFI et al., 1974), and if we are to be truly rigorous in our critique, then we have little unequivocal evidence that wheat gluten is lysine-limited for humans.
The most
comprehensive study in the literature is the MIT study (SCRIMSHAW
and YOUNG, 1973). In response to lysine supplementation of wheat
gluten-based diets, fed at two levels of protein and two levels
of energy, there were small reductions (2.9-7.7%) in urea
excretion which, although significant on a paired basis, were
remarkably small responses if lysine content does limit wheat
gluten utilisation. Indeed, the responses were so small that they
can be explained as a consequence of the experimental design. In
the nitrogen balance studies, lysine supplementation was
evaluated in individuals after they had been fed the
unsupplemented diet. Since nitrogen balance improves with time
due to adaptation (RAND et al., 1985), the small
improvement of nitrogen balance with the lysine supplementation
may have been such an improvement with time. In my view, the
assumption that wheat gluten is lysine-limited in human diets is
unproven. Specific examination of the nitrogen balance response
to lysine supplementation of wheat-based diets in young children
failed to show any response (REDDY, 1971). Furthermore,
examinations of nitrogen balance responses in several studies of
children fed mixed vegetarian diets (TORUN, YOUNG and RAND,
1981), did not indicate that dietary protein quality was a
determinant of nitrogen balance. However, as already indicated in
the comments relating to studies such as those shown in Figure
1, part of the problem relates to our limited ability to
understand nitrogen balance.
Perhaps the most
significant problem we have to deal with in our attempts to
rationalise IAA needs is posed by the evidence relating to urea
salvage in the lower gut. Urea hydrolysis in the gut has long
been known to occur, but the work of Jackson and colleagues
(JACKSON et al., 1990; LANGRAN et al., 1991)
suggests that not only is this regulated, but it may have
profound importance for IAA provision for the organism. The key
question is the extent to which the nitrogen salvaged from urea
is incorporated into amino acids synthesised de novo by
microflora and then recycled into the body amino acid pool.
In fact, our own studies of leucine and phenylalanine metabolism (MILLWARD et al., l991b) suggest the possibility of de novo synthesis of these two amino acids. We have shown that, in normal adults fed low-protein diets, the rate of leucine oxidation and phenylalanine hydroxylation, measured with 13C and 2H, is markedly in excess of what would be expected from the rate of nitrogen excretion. While methodological considerations cannot be ruled out, it is difficult to identify any explanation other than the de novo synthesis of IAAs from urea by bacteria in the lower gut.
The
implications of urea salvage and de novo synthesis of IAAs
are profound. The extent implied by our results with leucine on
the low-protein diet is a daily recycling of de novo synthesised
amino acids equivalent to 25% of the dietary intake, and a higher
fraction (at least 43%) for phenylalanine (MILLWARD et al.,
1991b). This makes the concept of protein quality meaningless,
with the potential for qualitative modification of the amino acid
balance by the lower gut, and allows for considerable adaptation.
Thus, minimum obligatory IAA requirements can only be judged to
be an open question.
As already indicated, there
is a substantial amount of circumstantial evidence to suggest
that height growth in children reflects the overall level of
protein intake (GOLDEN, 1985), and can be assumed therefore to be
one important target of the anabolic drive. However, few studies
have addressed the question of whether the amino acid quality of
dietary protein is important in this role. In one study from
South India, comparing the ability of different cereals to
support height growth over six months, children fed rice-based
diets (n = 10) grew in height at a faster rate than others fed
isonitrogenous, isoenergetic wheat-based diets (BEGUM et al.,
1970). On the basis of the amino acid compositions of the diets,
with lower levels of lysine threonine and isoleucine in the
cereal diets, the authors suggested that height growth was
limited by IAA supply. However, as pointed out by VAGHEFI et
al., (1974) in their review of this and other nutritional
studies with cereal-based diets, the possibility of other
nutrients limiting height growth must be considered, especially
since riboflavin deficiency was actually described in some of the
children in these studies. Indeed, GOLDEN, GOLDEN and BENNETT
(1988) have argued that in most nutritional studies relating to
child growth, even in the absence of any clear signs of
deficiencies which might occur on these cereal-based diets,
interpretation of the responses is often most difficult, because,
for several nutrients which limit growth when deficient, this
deficiency cannot be detected since no unique signs occur apart
from growth failure. Thus, GOLDEN et al., (1988) argue
that, in addition to protein, any one of the minerals such as
zinc, phosphate, potassium, magnesium and sodium could be
growth-limiting due to its low content and/or bioavailability.
Certainly, several supplementation studies in young children
(HAMBIDGE, 1991) have indicated an important role for zinc
deficiency in inadequate height growth in children.
The evaluation of the extent and mechanisms by which the anabolic drive of dietary protein and IAAs does regulate height growth is, in my view, a most important research task. In fact we know little about the relationship between IAA intakes and any regulatory responses which comprise the anabolic drive. One possibility relates to the role of amino acids in the regulation of the secretion of the anabolic hormones. It has long been known from studies on rodent pancreatic islet control, that the potency of amino acids as insulin secretagogues varies markedly (FAJANS and FLOYD, 1972) and that, with the exception of arginine (the most powerful secretagogue), the IAAs are generally more potent than the dispensable amino acids. Thus, an influence of protein quality on insulin secretion and consequent changes in the levels of the other anabolic hormones regulated by insulin, is one possible mechanism. However, given the fact that, in humans, insulin secretion is less dependent on amino acids than in rodents (see MILLWARD, 1990a), some caution is required in adopting such an explanation.
Animal
studies are not particularly informative since very few of them
have specifically looked at the regulatory influence of dietary
protein sources of different quality. In one study, CREE and
SCHALCH (1985) reported higher IGF-1 levels in rats fed casein,
compared with isonitrogenous wheat gluten. However, in the rat,
plasma IGF-1 levels (and insulin levels; JEPSON et al.,
1988) increase with increasing age and body weight and, since the
casein-fed rats grew faster, it was not possible to disentangle
the influences of body weight growth, as opposed to dietary
protein source, on the IGF-1 level.
My aim at the outset was to
explore the extent of our understanding of the metabolic basis of
the requirements for amino acids and protein, and to argue for a
more rational model of the organism's needs. Having elaborated
such a model, I am conscious of the likely response: "Where
does this get us, and how does this help in interpreting the data
in Figure 1?" If the model is of any value at all, it should
help in allowing better definition of protein requirements,
amount and quality, not least in young children.
In my view, the crucial point is recognition of the two levels of requirement - minimum and optimum. Most of our efforts to date, namely the N-balance data reviewed in the 1985 report (FAO/WHO/UNU, 1985), have focused on Rmin. The stable isotope metabolic balance studies (e.g., MEGUID et al., 1986) are in effect attempting to establish an Roperative which needs bear little or no relationship to either Rmin or Ropt. What they do establish, discounting any technical problems, is the leucine, valine, threonine and lysine intakes required to balance losses of these amino acids generated by a diet containing all other amino acids at a level equivalent to that of 0.8 g egg protein. The information from these and subsequent studies is important in advancing our understanding of amino acid interactions as influences on oxidation rates, a long-standing important metabolic question (HARPER and ELVEHJEM, 1955) but does not, in my view, help us better define nutritional requirements for protein.
I believe our task for the future is the determination of Ropt. The first step has in fact been taken in the recent publications of dietary reference values in the UK (UK Department of Health, 1991), by defining an upper limit for adults beyond which it may not be safe. Our task now is to agree on functional indicators of adequacy, i.e., targets of the anabolic drive, which enable us to define Lr opt, and hence Ropt. Such indicators are unlikely to be simple to measure. Height growth, immunocompetence, and the extent of urea recyling are potential indicators which all require considerable expertise and resources for their study, assuming they prove to be appropriate. However, in my view, without an investment in such studies, we are unlikely to be able to generate any new data of sufficient worth to warrant adjustment of the existing data. Thus, resolution of the dilemma posed by the data in Figure 1 requires measurements of the functional responses of these children to their protein intakes, measurements which may well take many years, but for which, in my view, there is no alternative solution.